Abstract
The objective of this study was to investigate the safety and performance of AQUACEL™ Ag+ dressing, a wound dressing containing a combination of anti‐biofilm and antimicrobial agents, in the management of chronic wounds. Patients (n = 42) with venous leg ulcers exhibiting signs of clinical infection were treated for 4 weeks with AQUACEL™ Ag+ dressing, followed by management with AQUACEL™ wound dressings for 4 weeks. Wound progression, wound size, ulcer pain and clinical evolution of the wound were assessed for up to 8 weeks. Adverse events were recorded throughout the study. AQUACEL™ Ag+ dressing had an acceptable safety profile, with only one patient discontinuing from the study, because of a non‐treatment‐related adverse event. After 8 weeks, substantial wound improvements were observed: 5 patients (11·9%) had healed ulcers and 32 patients (76·2%) showed improvement in ulcer condition. The mean ulcer size had reduced by 54·5%. Patients reported less pain as the study progressed. Notable improvements were observed in patients with ulcers that were considered to require treatment with systemic antibiotics or topical antimicrobials at baseline (n = 10), with a mean 70·2% reduction in wound area. These data indicate that AQUACEL™ Ag+ dressing has an acceptable safety profile in the management of venous leg ulcers that may be impeded by biofilm.
Keywords: Antimicrobial dressing, Biofilm, Venous leg ulcer, Wound healing
Introduction
Venous leg ulcers are chronic wounds associated with recalcitrance, pain and reduced quality of life for patients, as well as considerable cost to health care providers 1, 2. Such wounds are often resistant to treatment, and patients can typically experience ulcer durations of a year or more 3. Chronic wounds provide an ideal environment for wound microflora, and bacterial colonisation has been associated with delayed wound healing 4, 5, 6.
The failure of locally infected chronic wounds, and those at risk of infection, to respond to topical and systemic antimicrobial treatment is now considered to be associated with the presence of biofilm, which exists in a majority of chronic wounds and is associated with impaired wound healing 7, 8, 9. Biofilm is a complex, heterogeneous, polymicrobial community residing in a self‐produced matrix of polysaccharides, proteins and extracellular DNA, which can provide protection and increased tolerance to antimicrobial agents such as antibiotics and antiseptics, as well as the hosts' immune mechanisms 8, 10, 11. The presence of wound biofilm is most commonly determined by clinical assessment, and the key recognised indicators include wound surface slime or sheen, poor response to topical and systemic antimicrobial agents, excessive moisture, poor quality granulation tissue and history of persistent/recurring infection 12, 13, 14. There is increasing evidence that non‐healing wounds that are susceptible to recurrent infections and unresponsive to antimicrobial therapies are impeded because of the biofilm 12, 14.
To date, wound dressings designed to facilitate wound healing by specifically disrupting the biofilm and killing the associated micro‐organisms, to our knowledge have not been commercially available. AQUACEL™ Ag+ is a next‐generation antimicrobial dressing that has been developed to maximise the effectiveness of ionic silver against wound microorganisms in their natural, predominant and protective biofilm form. In addition to ionic silver, AQUACEL™ Ag+ dressing contains a metal chelating agent and a surfactant. The metal chelating agent assists the disruption of biofilm and the subsequent exposure of microorganisms to the action of ionic silver. The surfactant reduces surface tension and assists in loosening the biofilm structure. Both agents have been shown to act synergistically with ionic silver to considerably increase its antimicrobial activity in an in vitro biofilm model 15. This synergistic action in AQUACEL™ Ag+ dressing is intended to promote healing in previously recalcitrant, biofilm‐impeded wounds.
The purpose of this study was to evaluate the safety and performance of 4 weeks' use of AQUACEL™ Ag+ dressing followed by 4 weeks' use of AQUACEL™ dressing in patients with venous leg ulcers.
Methods
This was a pre‐market non‐comparative controlled study to assess the safety and performance of AQUACEL Ag+ dressing in the management of chronic venous leg ulcers. The Clinical Investigation Plan and supporting documentation were reviewed and approved by the appropriate country‐specific ethics committees and appropriate notified bodies. This study was carried out in accordance with ISO14155:2011 and the Declaration of Helsinki. All enrolled patients gave informed consent before any study procedures were performed.
Patients
A total of 42 patients with a venous leg ulcer [CEAP (clinical severity, aetiology, anatomy, pathophysiology) classification C6] were recruited. The inclusion criteria were: patients aged 18 years or over, with an ankle to brachial pressure index of 0·8 or greater and venous leg ulcers of less than 24 months' duration, area of the leg ulcer between 5 and 40 cm2 and meeting at least three of five pre‐defined clinical signs of infection (pain between two dressing changes, peri‐ulcer skin erythema/inflammation, oedema, malodour and heavy exudate). In addition, the ulcers were categorised at baseline as either clinically infected (defined as a wound that required the use of systemic antibiotics or topical antimicrobials) or not clinically infected (exhibiting some signs and symptoms of clinical infection, but not requiring antibiotic or topical antimicrobial treatment). Allocation to these two groups was based on the judgement of individual clinicians following consideration of the wound history and presentation at the time of enrolment, and the severity associated with each of the clinical signs of infection. Patients were not administered topical antibiotics or antimicrobials during the study. All patients agreed to wear compression therapy daily in combination with the trial dressing.
Exclusion criteria included a history of skin sensitivity to any components of the treatment dressings, treatment with systemic antibiotics or topical antimicrobial agents in the week prior to inclusion (with the exception of wounds that were considered to be clinically infected at baseline), malignant leg ulcers, recent diagnosis of deep vein thrombosis, venous surgery in the past 3 months, a progressive neoplastic lesion treated by radiotherapy or chemotherapy or ongoing treatment with immunosuppressive agents.
Study treatment
Patients who met the selection criteria and gave written informed consent to participate in the study were managed with the AQUACEL™ Ag+ dressing for 4 weeks, in line with standard practice for antimicrobial dressings 16, followed by 4 weeks of management with the non‐antimicrobial AQUACEL™ dressing. Dressings were applied to cover the whole surface of the ulcer, plus at least 1 cm overlap onto the peri‐ulcer skin for the 5 cm × 5 cm dressing or at least 2 cm overlap for larger dressings. The primary dressing was completely covered by a moisture‐retentive secondary dressing appropriate to the clinical condition of the wound and the volume of exudate. An appropriate compression system (minimum UK Class 3) was applied. Dressings were changed at least once a week by a health care professional. Wounds were assessed weekly until Week 4 and fortnightly thereafter. Patients were followed up until complete healing or for a maximum of 8 weeks until the end of the treatment period. Adverse events (AEs) were recorded throughout the study and coded according to the Medical Dictionary for Regulatory Activities (MedDRA) terms.
Performance evaluations
At each study visit, the condition of the wound and the peri‐ulcer skin was assessed, photographed and traced. The overall condition of the ulcer was categorised as either healed, marked improvement, mild improvement, no change, deterioration or marked deterioration. Ulcer pain in the patients was recorded using the Johns Hopkins visual analogue scale (VAS; where 0 = no pain and 10 = worst pain imaginable). Patient comfort during dressing changes and investigators' assessment of dressing performance were also recorded. Changes in performance assessments from baseline to final evaluation or throughout the 8‐week study were summarised using descriptive statistics (frequencies and percentages). Comparison of categorical variables was performed by χ2 test, and continuous variables were compared by analysis of variance (ANOVA). Data analysis was performed using SAS® version 9.2 (SAS Institute Inc., Cary, NC).
Results
Study patients
A total of 42 patients were enrolled at six study centres in the UK and Poland. Of these, 10 patients had ulcers at baseline that were considered by a clinician to require the use of systemic antibiotics or topical antimicrobial agents (clinically infected). Five ulcers (11·9%) healed completely prior to the end of the study. A further 36 patients completed the full 8‐week study duration. One patient (2·4%) discontinued from the study, because of a serious adverse event (SAE) that was considered not related to the study treatment (fractured femur). The baseline demographics of the study population are summarised in Table 1.
Table 1.
Baseline demographics of the study population
| Baseline characteristics by treatment group | |||
|---|---|---|---|
| Clinically infected (n = 10) | Not clinically infected (n = 32) | Total (n = 42) | |
| Gender, n (%) | |||
| Male | 6 (60·0) | 10 (31·3) | 16 (38·1) |
| Female | 4 (40·0) | 22 (68·8) | 26 (61·9) |
| Age (years) | |||
| Mean ± SD | 65·6 ± 13·3 | 73·2 ± 11·9 | 71·4 ± 12·5 |
| Median | 64·0 | 76·0 | 74·5 |
| Min, Max | 41·0, 88·0 | 36·0, 92·0 | 36·0, 92·0 |
| Duration of ulcer (years) | |||
| Mean ± SD | 1·11 ± 0·86 | 0·83 ± 0·53 | 0·91 ± 0·65 |
| Median | 1·17 | 0·75 | 0·75 |
| Min, Max | 0·08, 2·00 | 0·08, 1·92 | 0·08, 2·00 |
| Ulcer status, n (%) | |||
| Recurrent | 6 (60·0) | 17 (53·1) | 23 (54·8) |
| New | 4 (40·0) | 15 (46·9) | 19 (45·2) |
| Superficial | 0 | 11 (34·4) | 11 (26·2) |
| Shallow | 8 (80·0) | 20 (62·5) | 28 (66·7) |
| Deep | 2 (20·0) | 1 (3·1) | 3 (7·1) |
| Ulcer condition, n (%) | |||
| Improving | 2 (20·0) | 10 (31·3) | 12 (28·6) |
| No progress | 2 (20·0) | 13 (40·6) | 15 (35·7) |
| Deteriorating | 6 (60·0) | 9 (28·1) | 15 (35·7) |
SD, Standard deviation.
Demographic characteristics were generally comparable between the patient groups, although the mean age and proportion of female patients were lesser in the clinically infected group (Table 1). Ulcer recurrence was a common trait in the population of patients studied, with an overall 54·8% of the reference ulcers having recurred. Notably, for most patients (60·0%) in the clinically infected group, the ulcer was considered to be deteriorating at baseline, compared to 28·1% of patients in the non‐infected group. In both groups, the majority of ulcers (80·0% and 68·7% in the clinically infected and non‐infected groups, respectively) were either not progressing or deteriorating at baseline (Table 1). Ulcer durations at baseline varied between 1 month and 2 years. Median ulcer durations were 1·17 and 0·75 years in the clinically infected and non‐infected groups, respectively (Table 1), suggesting a degree of wound recalcitrance [considered an indicator of biofilm presence; 12, 14] in both study groups. At baseline, 9 patients (90·0%) in the clinically infected group and 27 patients (84·4%) in the non‐infected group were being treated with compression therapy.
Given the differences in the ulcer condition between patient groups, and the different approach to antimicrobial treatment prior to study entry (treatment with systemic antibiotics or topical antimicrobial agents in the week prior to inclusion was prohibited in the non‐infected group only), performance data for these patient groups was considered separately.
Safety and tolerability
Overall, the treatment regimen was well tolerated. A total of 28 patients (66·7%) experienced AEs during the use of AQUACEL™ Ag+ dressing and 17 patients (40·5%) experienced AEs during the use of AQUACEL™ dressing. The most commonly reported AEs were pain, wound decomposition, venous ulcer pain and bloody discharge, which were all consistent with the patient population. All reported AEs of wound decomposition corresponded to an increase in ulcer size. All AEs considered related to study treatment are presented in Table 2. Overall, 15 patients (35·7%) and 8 patients (19·0%) had AEs that were considered related to AQUACEL™ Ag+ and AQUACEL™ dressings, respectively. The most commonly reported related AE was pain, which was experienced by five (11·9%) and two (4·8%) patients during the use of AQUACEL™ Ag+ and AQUACEL™ dressings, respectively. All other treatment‐related AEs were experienced by less than 10% of patients.
Table 2.
Adverse events considered related to study treatment
| MedDRA preferred term | Adverse events, n (%) | |
|---|---|---|
| AQUACEL Ag+™ (Weeks 1–4) | AQUACEL™ (Weeks 4–8) | |
| Total patients with an event considered related to study treatment | 15 | 8 |
| Pain | 5 (11·9) | 2 (4·8) |
| Wound decomposition | 2 (4·8) | 3 (7·1) |
| Bloody discharge | 3 (7·1) | 0 |
| Skin maceration | 3 (7·1) | 0 |
| Wound complication | 2 (4·8) | 0 |
| Erythema | 1 (2·4) | 1 (2·4) |
| Wound haemorrhage | 1 (2·4) | 1 (2·4) |
| Burning sensation | 1 (2·4) | 0 |
| Eczema | 1 (2·4) | 0 |
| Peripheral oedema | 1 (2·4) | 0 |
| Rash | 1 (2·4) | 0 |
| Skin disorder | 1 (2·4) | 0 |
| Venous ulcer pain | 1 (2·4) | 0 |
| Increased fibrin | 0 | 1 (2·4) |
| Pruritis | 0 | 1 (2·4) |
MedDRA, Medical Dictionary for Regulatory Activities.
Two patients experienced SAEs during the course of the study. One patient had a fractured femur, which resulted in treatment discontinuation and withdrawal from the study. The second patient had a fractured right hip. Neither of these SAEs were considered related to study treatment.
Overall, eight patients (80·0%) in the clinically infected group and five patients (15·6%) in the non‐infected group received concomitant antibiotics. All of the eight patients from the clinically infected group received antibiotics for indications of wound or ulcer infections. As antibiotic treatment was permitted in the week prior to enrolment for these patients, it is likely that this reflects baseline antibiotic usage. All five patients in the non‐infected group who received antibiotics were prescribed their use as treatment for an AE. Only one of these patients received treatment for infection of the study wound.
Wound progression
At baseline, 30 patients (71·4%) had ulcers that were either deteriorating or not improving. Overall, by Week 8, complete healing was observed in 5 of the 41 evaluable patients (12·2%), while 24 patients (58·5%) showed marked improvement and 8 patients (19·5%) mild improvement in ulcer condition from baseline. Only one patient showed marked deterioration from baseline, while another patient showed mild deterioration from baseline. The remaining two patients who completed the study had no change in ulcer condition. All 10 patients with clinically infected ulcers showed some degree of improvement (Figure 1), one patient (10·0%) had a healed ulcer, and 8 patients (80·0%) showed marked improvement in ulcer condition from baseline.
Figure 1.
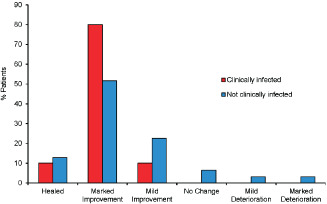
Progression in wound condition from baseline. Overall change in ulcer condition was assessed from baseline to the end of the study in patients with clinically infected ulcers (n = 10, red bars) or non‐infected ulcers (n = 31, blue bars).
The mean ulcer size decreased in both the clinically infected group (n = 10; Figure 2A) and the non‐infected patient group (n = 32). Representative images of ulcer area reduction in patients whose ulcers healed are presented in Figure 2B. After 8 weeks, the mean reduction in ulcer area for the whole population was 54·5% (standard deviation: 42·8%). Although the mean ulcer area relative to baseline in the 10 patients with clinically infected ulcers increased at Week 1, all of these patients experienced a reduction in ulcer area by Week 8. The mean ulcer area reduction in patients with clinically infected ulcers was 70·2% (standard deviation: 24·7%, range: 13·6–100%; Figure 2A).
Figure 2.
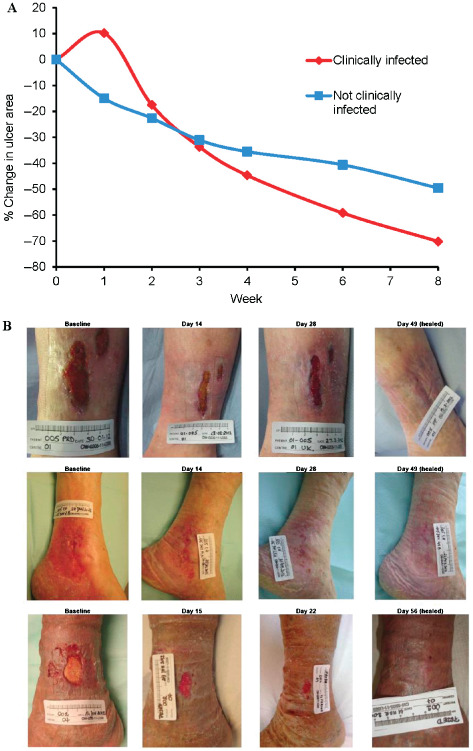
Reduction in ulcer size. (A) Mean percentage reduction in ulcer area was measured at each treatment visit for patients with clinically infected ulcers (n = 10, red line) or non‐infected ulcers (n = 32, blue line). (B) Progression of ulcer area reduction in three patients with healed ulcers at the end of the study. Patients gave prior written consent for photographs to be published.
Pain
Patient‐reported assessment of pain, based on a VAS where 0 = no pain and 10 = worst pain imaginable, showed that the mean level of ulcer pain decreased from 4·46 at baseline to 2·98 at Week 4 and 1·94 at Week 8. In addition, over time, fewer patients reported moderate or severe pain between dressing changes compared with baseline [15 patients (36·6%) and 9 patients (22·5%] at Week 4 and Week 8, respectively, compared to 27 patients (64·3%) at baseline].
Dressing performance
Overall dressing performance with the AQUACEL™ Ag+ dressing was highly favourable for ease of use, conformability and the ability of the dressing to remain intact during wear and on removal.
Discussion
AQUACEL™ Ag+ is a next‐generation antimicrobial dressing that has been designed to maximise antimicrobial effectiveness by exposing microorganisms existing in their natural, predominant and protected biofilm form to ionic silver. The aim of this pre market non‐comparative study was to provide safety and performance data to support the use of the dressing in the treatment of chronic venous leg ulcers with clinical signs indicative of the presence of biofilm. The data presented demonstrate that the use of AQUACEL™ Ag+ dressing for 4 weeks, followed by 4 weeks use of AQUACEL™ dressing under standard compression therapy, resulted in improvements in wound characteristics and reduction in wound size and perceived pain. These improvements were observed in patients with clinically infected ulcers, as well as in those with ulcers that were not considered to be clinically infected by the investigating clinician. The safety profile of the new dressing was consistent with that of the standard AQUACEL™ dressing.
The treatment regimen investigated in this study was intended to reflect anticipated clinical practice with AQUACEL™ Ag+ dressing. There are no formal published guidelines on the duration of antimicrobial dressing use. In a retrospective analysis of 3084 patients receiving antimicrobial wound dressings at 26 centres in the USA, the mean duration of the use of such dressings was 32·5 days [median 21 days; 16]. This practice is reflected in other clinical studies investigating antimicrobial wound dressings, where patients were managed with antimicrobial dressings for 4 weeks, followed by equivalent non‐antimicrobial dressings for a further 4 weeks 17, 18. The treatment regimen used in this study therefore reflects the current clinical practice for antimicrobial dressings and is consistent with other clinical studies investigating the performance of antimicrobial dressings. Importantly, improvements observed after 4 weeks of using AQUACEL™ Ag+ dressing continued during use of the non‐antimicrobial AQUACEL™ dressing. This finding indicates that 4 weeks use of AQUACEL™ Ag+ dressing is appropriate to promote healing of venous leg ulcers, as improvements were sustained even after the dressing was removed.
The AEs reported were consistent with those expected for the study population. Fewer AEs were reported during the use of the AQUACEL™ dressing than during the use of AQUACEL™ Ag+ dressing, which may reflect the wound healing observed during Weeks 1–4 (Figures 1 and 2). There were no SAEs considered related to study treatment, and the most frequently reported AE was pain; eight patients during use of AQUACEL™ Ag+ dressing and three patients during the use of AQUACEL™ dressing. Despite the relatively high frequency of pain reported as an AE, the mean pain score recorded by the patients decreased during the use of both the AQUACEL™ Ag+ dressing (from 4·46 to 2·98) and AQUACEL™ dressing (from 2·98 to 1·94). In addition, fewer patients reported moderate or severe pain between dressing changes throughout the study relative to baseline. These data suggest that the treatment regimen used in this study is beneficial in reducing the pain reported by patients with venous leg ulcers, as well as in supporting their healing.
The improvements in wound healing reported in this study are particularly encouraging given the baseline wound characteristics. Mean ulcer duration in all patients was 0·91 years, with only 28·6% of the patients' ulcers improving at baseline (Table 1). However, by Week 8, five patients had ulcers that had healed completely, and the majority (86·5%) of the remaining patients had ulcers that showed improvement from baseline and the mean ulcer size had reduced (Figures 1 and 2). Most of the patients in this study were already receiving compression therapy at baseline, in line with current evidence‐based recommendations for the management of venous leg ulcers 19. The improvements observed in the patients in this study (most of whom had non‐healing or deteriorating ulcers at baseline) are therefore likely to reflect the performance of the primary dressings investigated.
The improvements observed in patients with ulcers that were considered by the clinician to require antibiotic treatment were particularly notable. The difficulties in developing universal criteria for wound infection are acknowledged, and the presence or absence of specific signs does not always equate to wound infection 20, 21. There is evidence that traditional signs of clinical infection are often poor predictors of chronic wound infection 22. Clinicians' judgement was therefore used to assess which patients had clinically infected ulcers that would require treatment with antibiotics, in line with routine clinical practice 21. At baseline, 60·0% of the patients had deteriorating ulcers (Table 1). However, all patients with clinically infected ulcers had improvements in wound condition by Week 8 (Figure 1). Although the design of the study and the differences in baseline ulcer condition of the two treatment groups do not allow for comparison of the performance of the treatment between the groups, the observation that improvements in wound condition and size were greater in patients with clinically infected ulcers suggest that AQUACEL™ Ag+ dressing could be particularly suitable for these patients. The treatment of infected/recalcitrant wounds places great demands on health care resources. In a recent methodology proposed for evaluating the costs involved in wound care, treatment costs increased markedly with ulcer severity 23. Thus, the cost for treating deteriorating or severe ulcers were found to be between two and six times more per week than that for an ulcer that was progressing normally towards healing. Such costs reflected more frequent visits and hospitalisation for ulcers with severe complications 23. There is a requirement for dressings such as AQUACEL™ Ag+ dressing, which has demonstrated synergistic activity against biofilm in vitro 15 to address the challenging issues leading to recalcitrance in such wounds.
This study did not directly assess the microbiological characteristics of patient wounds, and the infection status of individual wounds was based on the clinician's assessment. Most of the wounds included in this study were recalcitrant, demonstrated signs of local infection and were unresponsive to previous local treatments, all considered to be characteristics of biofilm involvement 12, 14. Unambiguous identification of wound biofilm requires specialist microscopy techniques, which are impractical in the clinical setting 8, 9. In clinical practice, therefore, physician's assessment of biofilm presence is usually based on an assessment of several visual and clinical signs 12, 14. The population of patients included in this study, who presented with several signs indicative of biofilm presence, was therefore appropriate to assess the clinical use of a next‐generation wound dressing designed to have enhanced activity against wound biofilm.
Limitations
Acknowledging the small sample size in this study, statistical analysis was limited to frequencies, percentages and standard deviations, although comparison of categorical variables was performed by χ2 test, and continuous variables were compared by analysis of variance (ANOVA). Given both the small sample size and the non‐comparative nature of this study, larger, randomised controlled clinical studies are warranted to establish the true performance of AQUACEL™ Ag+ dressing in promoting healing in previously recalcitrant, biofilm‐impeded wounds.
Conclusions
The data presented in this study indicate that a regimen of 4 weeks use of AQUACEL™ Ag+ dressing followed by 4 weeks use of AQUACEL™ dressing promoted healing in previously slow‐healing, recalcitrant or deteriorating venous leg ulcers. Substantial improvements were reported in patients with both infected and non‐infected ulcers at baseline. The dressing demonstrated a satisfactory safety profile with only two patients experiencing SAEs, neither of which was considered related to study treatment. No patient experienced a treatment‐related AE that led to withdrawal from the study. AQUACEL™ Ag+ dressing, a wound dressing with demonstrated synergistic efficacy against biofilm in vitro, provides a promising option for facilitating wound healing in patients with potentially recalcitrant, difficult to treat, chronic venous leg ulcers.
Acknowledgements
This study was supported by ConvaTec Inc, manufacturers of AQUACEL™ dressings and AQUACEL™ Ag+ dressings. Editorial support for the preparation of the manuscript was provided by Insight Medical Writing (Oxford, UK).
JC, KP, DP and PB are employees of ConvaTec Inc, and DP is named as an inventor on a patent relating to AQUACEL™ Ag+. None of the authors was in receipt of any other funding that they believe represents a potential conflict of interest for this study.
References
- 1. Simon DA, Dix FP, McCollum CN. Management of venous leg ulcers. Br Med J 2004;328:1358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hopman WM, Buchanan M, Van Den Kerkhof EG, Harrison MB. Pain and health‐related quality of life in people with chronic leg ulcers. Chronic Dis Inj Can 2013;33:167–74. [PubMed] [Google Scholar]
- 3. Moffatt CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulceration in a London population. QJM 2004;97:431–7. [DOI] [PubMed] [Google Scholar]
- 4. Bowler PG, Davies BJ. The microbiology of infected and noninfected leg ulcers. Int J Dermatol 1999;38:573–8. [DOI] [PubMed] [Google Scholar]
- 5. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halbert AR, Stacey MC, Roht JB, Jopp‐McKay A. The effect of bacterial colonization on venous ulcer healing. Australas J Dermatol 1992;33:75–80. [DOI] [PubMed] [Google Scholar]
- 7. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–22. [DOI] [PubMed] [Google Scholar]
- 8. James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 9. Gurjala AN, Geringer MR, Seth AK, Hong SJ, Smeltzer MS, Galiano RD, Leung KP, Mustoe TA. Development of a novel, highly quantitative in vivo model for the study of biofilm‐impaired cutaneous wound healing. Wound Repair Regen 2011;19:400–10. [DOI] [PubMed] [Google Scholar]
- 10. Høiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen PØ, Molin S, Givskov M, Tolker‐Nielsen T, Bjarnsholt T. The clinical impact of bacterial biofilms. Int J Oral Sci 2011;3:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill KE, Davies CE, Wilson MJ, Stephens P, Harding KG, Thomas DW. Molecular analysis of the microflora in chronic venous leg ulceration. J Med Microbiol 2003;52:365–9. [DOI] [PubMed] [Google Scholar]
- 12. Metcalf DG, Bowler PG, Hurlow J. A clinical algorithm for wound biofilm identification. J Wound Care 2014;23:137–42. [DOI] [PubMed] [Google Scholar]
- 13. Hurlow J, Bowler PG. Potential implications of biofilm in chronic wounds: a case series. J Wound Care 2012;21:109–19. [DOI] [PubMed] [Google Scholar]
- 14. Metcalf DG, Bowler PG. Clinician perceptions of wound biofilm. Int Wound J 2014. DOI: 10.1111/iwj.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Said J, Walker M, Parsons D, Stapleton P, Beezer AE, Gaisford S. An in vitro test of the efficacy of an anti‐biofilm wound dressing. Int J Pharm 2014;474:177–81. [DOI] [PubMed] [Google Scholar]
- 16. Fife CE, Carter MJ, Walker D, Thomson B. A retrospective data analysis of antimicrobial dressing usage in 3,084 patients. Ostomy Wound Manage 2010;56:28–42. [PubMed] [Google Scholar]
- 17. Harding K, Gottrup F, Jawień A, Mikosiński J, Twardowska‐Saucha K, Kaczmarek S, Sopata M, Shearman C, Pieronne A, Kommala D. A prospective, multi‐centre, randomised, open label, parallel, comparative study to evaluate effects of AQUACEL® Ag and Urgotul® Silver dressing on healing of chronic venous leg ulcers. Int Wound J 2012;9:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazareth I, Meaume S, Sigal‐Grinberg ML, Combemale P, Le Guyadec T, Zagnoli A, Perrot J‐L, Sauvadet A, Bohbot S. The role of a silver releasing lipido‐colloid contact layer in venous leg ulcers presenting inflammatory signs suggesting heavy bacterial colonization: results of a randomized controlled study. Wounds 2008;20:158–66. [PubMed] [Google Scholar]
- 19. Grey JE, Enoch S, Harding KG. Venous and arterial leg ulcers. Br Med J 2006;332:347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Wound Management Association (EWMA) . Position document: identifying criteria for wound infection. London: MEP Ltd., 2005. [Google Scholar]
- 21. World Union of Wound Healing Societies (WUWHS) . Principles of best practice: wound infection in clinical practice. An international consensus. London: MEP Ltd., 2008. [Google Scholar]
- 22. Nelson EA, O'Meara S, Craig D, Iglesias C, Golder S, Dalton J, Claxton K, Bell‐Syer SEM, Jude E, Dowson C, Gadsby R, O'Hare P, Powell J. A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers. Health Technol Assess 2006;10:iii–iv, ix–x, 1–221. [DOI] [PubMed] [Google Scholar]
- 23. Harding K, Posnett J, Vowden K. A new methodology for costing wound care. Int Wound J 2013;10:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


