Fig. 1.
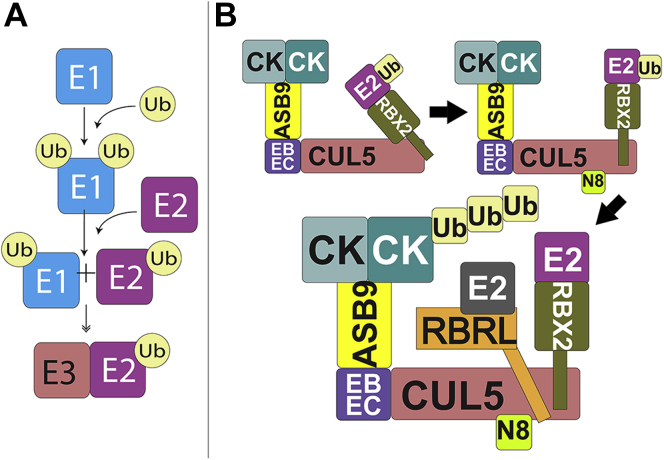
Schematics of the ubiquitylation cascade as mediated by the ASB9 CUL5 E3 ligase.A, ubiquitin is activated through a three-step enzymatic cascade. The ubiquitin-activating enzyme (E1) binds ATP and catalyzes adenylation of ubiquitin. The active-site cysteine on E1 attacks the Ub-AMP complex to form a thioester bond. Through a trans-thioesterification reaction, ubiquitin is transferred to the active-site cysteine on a ubiquitin-conjugating enzyme (E2). Ubiquitin ligases (E3) facilitate the highly specific covalent attachment of activated ubiquitin (Ub) to bound substrate proteins through an isopeptide bond on an exposed lysine residue. B, schematic showing the states of the ASB9-CRL explored in this work: unactivated, activated by NEDD8, and activated by NEDD8 with ARIH2 present. The protein abbreviations and colors are consistent throughout the manuscript: creatine kinase brain-type (CKB, two-tone teal); ankyrin and SOCS-box protein 9 (ASB9, yellow); elongins B and C (ELOB/C, purple); cullin 5 (CUL5, salmon), ring box protein 2 (RBX2, olive); the ubiquitin conjugating enzyme bound to RBX2 (UBE2D1/2, magenta); ubiquitin (Ub, tan); Ariadne RBR E3 ubiquitin protein ligase 2 Ring between Ring ligase (ARIH2 RBRL, orange); the ARIH2 RBRL ubiquitin conjugating enzyme (UBE2L3, gray). The Ub is transferred from UBE2L3 to ARIH2 prior to transfer to the substrate.