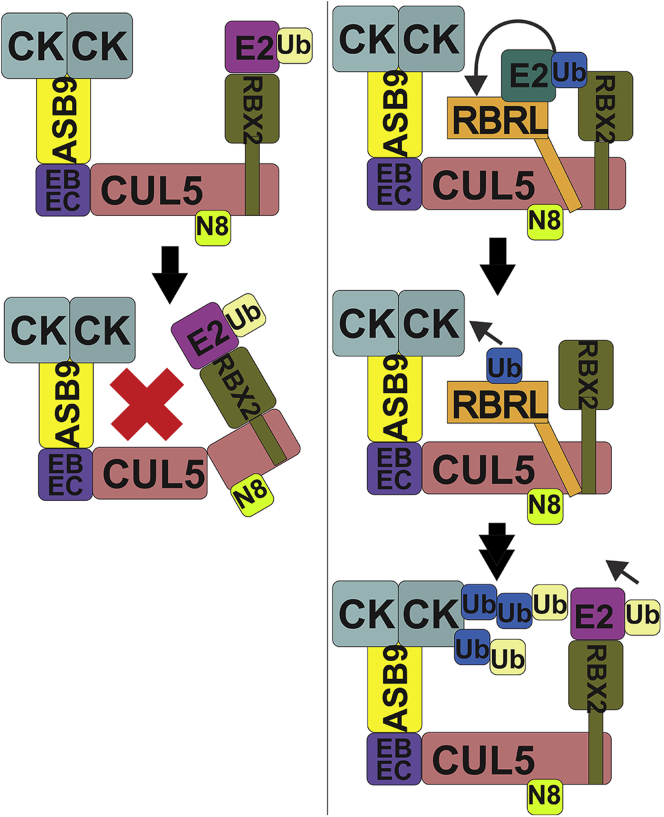
Fig. 8.
Schematic diagram of the CRL E3 ligase mechanism based on the results of this study. Our data support a mechanism by which RBX2 initially engages UBE2F to neddylate CUL5. Neddylation alters the RBX2 to decrease exchange at its binding site for UBE2F. Neddylation also alters the conformation of CUL5 opening a cleft of basic residues to which the acidic C terminus of ARIH2 can bind with high affinity. ARIH2 is autoinhibited, but upon binding to CUL5, its active site increases exchange via a long-range allosteric mechanism. The increased exchange likely indicates opening of the ARIH2 active site and relief of autoinhibition. After initial Ub transfer by ARIH2 to specific lysines on CKB that are close to the ARIH2 active site, polyubiquitylation on those same lysines can occur by a number of different routes including continued ubiquitylation by ARIH2 or contributions from UBE2D2 or UBE2R1 bound to RBX2.