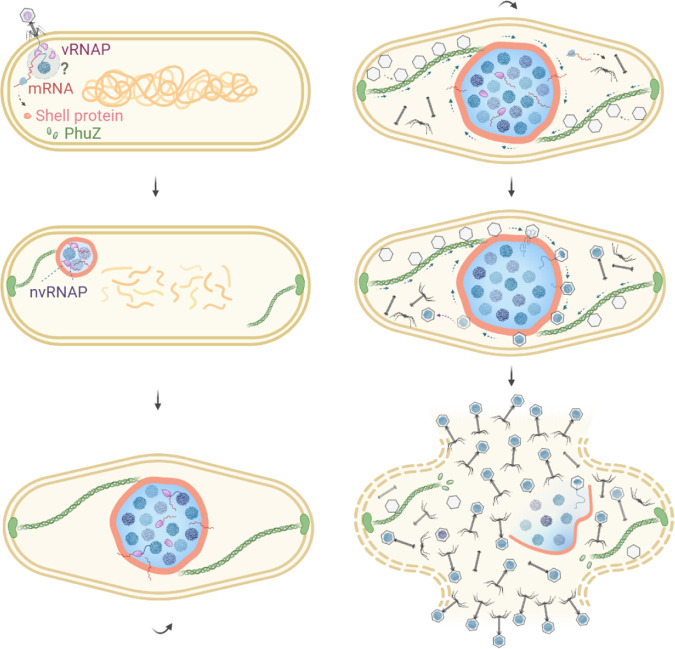
FIG 3.
Intracellular development of jumbo phages after infection of a bacterial host. Upon encountering a bacterial host, the phage injects its genomic DNA, accompanied by the translocation of virion RNA polymerases (vRNAPs) that are present inside the viral capsid. vRNAPs transcribe early phage genes, including genes encoding the shell protein and PhuZ. While the host chromosome is degraded, PhuZ forms filaments anchored at each cell pole and gradually establishes a bipolar spindle in the cell. Meanwhile, the shell proteins assemble a nucleus-like compartment to sequester phage DNA from the cytoplasm and protect phage DNA against host defense systems. Nonvirion RNAPs (nvRNAPs) appear later inside the compartment and are responsible for expression of the late phage genes, including viral structural genes. As phage DNA replicates inside the shell, the compartment grows and is pushed toward the cell center by dynamically unstable filaments of the PhuZ spindle. The cell becomes elongated and forms a bulge at midcell. After the phage nucleus is settled in the cell center, treadmilling PhuZ filaments rotate the shell locally and deliver empty phage capsids from the cell inner membrane to the phage nucleus for subsequent DNA encapsidation. Rotation of the phage nucleus facilitates even distribution of phage capsids around the shell surface, ensuring efficient DNA packaging. Eventually, filled capsids are released from the shell and assemble into mature phage particles together with other viral structural components that are generated in the cytoplasm, followed by cell lysis to release phage progeny to the environment.