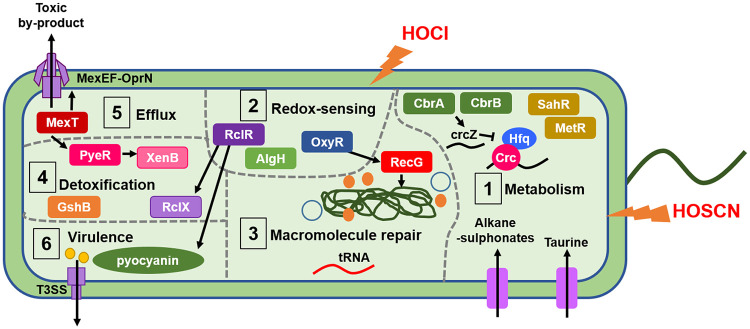
FIG 10.
Model of the putative defense mechanisms used by P. aeruginosa in response to HOCl and HOSCN stress. In step 1, P. aeruginosa responds to HOCl and HOSCN by upregulation of sulfur transport and metabolism genes; this may facilitate increased uptake of alternative sulfur sources, including taurine, to replenish depleted sulfur levels caused by HOCl and HOSCN oxidative damage. Methionine biosynthesis and metabolism regulators MetR and SahR appear required to protect against HOCl, which we postulate is a response to oxidative disruption of the metabolism of the sulfur-containing amino acid methionine and the cellular need to maintain its levels. Catabolite repression by the Crc protein appears required for protection against HOCl, possibly indicating that optimal regulation of metabolic flexibility aids defense against HOCl. In step 2, the transcriptional regulator RclR, the H2O2 sensor OxyR (42), and the hypothetical protein AlgH (41) all have putative redox-sensing capabilities and were identified as playing a role in protection against HOCl. In step 3, macromolecular repair mechanisms are implicated in the response to HOCl and HOSCN. Upregulation of tRNAs in response to HOCl may occur to replace oxidatively damaged tRNA. The DNA repair enzyme RecG is required for protection against HOCl. In step 4, putative bacterial detoxification enzymes are involved in the response to HOCl and HOSCN. The PyeR-regulated oxidoreductase XenB is induced in response to HOCl and is required for protection, and the glutathione synthetase GshB is required for protection against HOCl. The peroxiredoxin RclX is positively regulated by RclR in response to HOCl and HOSCN and required for protection against both oxidants. In step 5, upregulation of the MexT-regulated mexEF-oprN operon, encoding a multidrug efflux pump (28, 29), occurs in response to HOCl and HOSCN and is required for protection against HOCl. This highlights a link between oxidative stress and antibiotic resistance and indicates a role for the efflux pump in expelling toxic by-products of these oxidants. In step 6, RclR is required for survival against HOCl and HOSCN and regulates a number of genes in the presence of both oxidants, including upregulation of the phz genes, which synthesize the virulence factor pyocyanin (52). The genes responsible for the biogenesis and regulation of another virulence mechanism, the T3SS (51), are upregulated in response to HOCl and HOSCN. Induction of pyocyanin and the T3SS may occur as a mechanism to counterattack the innate immune cell-derived HOCl and HOSCN-mediated oxidative attack on the bacteria.