Abstract
Increasing data suggesting that microorganisms in the biofilm form are among the leading agents of persistent infections of chronic wounds require the development of new approaches to treatment. The aim of this article was to compare the efficacy of three commonly used antiseptics using a biofilm‐oriented approach. Biofilm‐oriented antiseptics test (BOAT), the innovative method, allows to estimate, in a quick and reliable manner, the in vitro activity of working solutions of antiseptics in real contact times against bacteria in the biofilm form and to use the results in the selection of an appropriate antiseptic to treat local infections in the clinical practice.
Keywords: Antiseptics, Biofilm‐oriented approach, P. aeruginosa, S. aureus
Introduction
It is estimated that at least 80% of nosocomial infections are related to the presence of biofilm, a community of microorganisms adhered to surfaces and covered with external layers of slime, protecting the bacteria not only from physical and chemical factors but also from immune system components 1. Biofilm‐forming bacteria are aetiological factors of many types of infections of chronic wounds, skin and soft tissues as well as of biomaterial‐related (dressings, stents, catheters, mesh, etc.) infections 2. From the clinical point of view, the most important attribute of biofilm is its very high resistance to antimicrobials, especially antibiotics. As this resistance was proven and was a satisfactory explanation of why many antibiotic treatment cases were ineffective, it became clear that more stress should be placed on other methods of bacterial eradication. The new biofilm‐oriented approach used to treat infected chronic wounds, referred to as biofilm‐based wound care 3, 4, 5, emphasises the necessity of frequent surgical debridement, the use of ‘anti‐biofilm’ agents and the use of selective biocides (antiseptics with broad antimicrobial spectrum and high tissue tolerability). Antiseptics used for local treatment of infections can be used in high, effective concentrations, thus displaying higher antimicrobial efficacy in comparison to local antibiotic therapy. Moreover, because the mechanisms of action of a majority of the modern antiseptics (PVP‐iodine and octenidine) excludes the possibility of bacterial resistance 6, it can be predicted that if the trend of growing occurrence of multi‐ and pan‐antibiotic‐resistant nosocomial strains does not subside, antisepsis will play a more important role in the treatment of local infections. Hence, although the new biofilm‐based approach to wound care is applied more frequently, so far there has been no simply, targeted method proposed to estimate the efficacy of antiseptics against bacteria in biofilm form. The existing methods are either planktonic‐oriented or require the use of specialised techniques or are so time‐ and work‐consuming that their introduction to the daily routine of standard‐equipped hospital laboratories appears to be impossible 7.
Therefore, the aim of this study was to develop a repeatable, cheap and simple biofilm‐oriented test that can be used to estimate the sensitivity of bacteria in the biofilm form to locally applied antiseptics.
Materials and methods
Bacterial strains
For the experiment purposes, 14 nosocomial strains of Pseudomonas aeruginosa and 14 strains of Staphylococcus aureus isolated from leg ulcer infections were chosen. In addition, reference ATTC S. aureus 6538 and ATTC P. aeruginosa 15445 were used.
Antiseptics
The following antiseptics were chosen for the experiment purposes:
Octenisept ® (Schulke & Mayr, Norderstedt, Germany): 100 g solution contains pharmaceutically effective ingredients: 0·1 g octenidine dihydrochloride and 2·0 g phenoxyethanol (Ph. Eur.). Other ingredients: (3‐coco fatty acid amidopropyl)‐dimethylazaniumylacetate, sodium‐d‐gluconate, glycerol 85%, sodium chloride, purified water and sodium hydroxide.
Rivanol ® (Polfa, Rzeszow, Poland): 100 g product contains pharmaceutically effective ingredient: 1 mg of ethacridine lactate; other ingredients: purified water to 100 g.
Braunol ® (Braun, Sempach, Switzerland): 100 g solution contains pharmaceutically effective ingredient: 7·5% povidone‐iodine; other ingredients: purified water, sodium biphosphonate, sodium iodide, macrogol lauryl ether and sodium hydroxide.
Biofilm‐oriented antiseptics test (BOAT) measuring antiseptics activity against bacterial biofilm
The strains were cultured into an appropriate liquid medium (TSB for P. aeruginosa and BC for S. aureus) and incubated at 37°C for 24 hours.
After incubation, the bacterial suspension was diluted with fresh medium to reach 1 × 105 cells/ml, and optical density was measured using a densitometer (Biomerieux, Warsaw, Poland).
Subsequently, 3× 100 µl of the suspension (1 × 105 cells) of an individual bacterial strain was transferred to three adjacent wells of a 96‐well polystyrene plate. This procedure was performed in duplicate (plate A and plate B).
Next, the suspensions were incubated at 37°C for 24 hours. After 24 hours, the suspensions from both plates were removed and thoroughly rinsed with 0·9% NaCl.
Next, (plate A) 100 µl of undiluted (working solution) of antiseptic was transferred to the well for selected contact time (1, 15 and 30 minutes). After the contact time, the antiseptic was removed and the wells were filled with an appropriate universal neutralising agent (Saline Peptone Water, Biocorp, Warsaw, Poland) for 5 minutes. After this time, the neutralising agent was removed.
The wells were filled with 100 µl of an appropriate medium and with 5 µl of tetrazolium chloride (TTC) (Fluka, Poznan, Poland), a reagent staining metabolically active microorganisms red. The results were assessed colorimetrically after 24 hours of incubation of the plate at 37°C.
In the case of plate B, stages 1–6 were performed in the same manner as in the case of plate A, whereas stage 5 was not performed. Plate B was used as a control of the strains' ability to form biofilm.
BOAT validation
Confirmation of the presence of biofilm on the bottom of polystyrene wells using electron microscopy
Polystyrene discs (Sarstedt, Stare Babice, Poland) of a diameter identical to the diameter of the wells were put on the bottom of a 32‐well polystyrene plate. Then, reference strains were incubated as described above (stages 1–3) and transferred to the disc‐containing wells and incubated for 24 hours at 37°C. After incubation, the discs were thoroughly rinsed with physiological saline to remove non‐adhered or weakly adhered bacterial cells from the material surface. Next, the discs were dried at 37°C for 6 hours. After drying, the surface of the discs was covered with Au/Pd (60:40) using Q150R ES device and examined in an electron microscope (ZEISS EVO MA 25) to confirm the presence of adhered cells and extracellular layers of slime.
Quantitative measurements of the number of colony‐forming units (BOAT version B) to prove the results obtained using qualitative results (BOAT version A)
One S. aureus and one P. aeruginosa strain, 4P and 2023, respectively, were incubated with the polystyrene discs as described above. The number of cells/control polystyrene disc (disc untreated with antiseptics) of the tested S. aureus and P. aeruginosa strain was 4·9 × 106 and 7·2 × 108, respectively. Next, antiseptics were added as described above (stages 1–5). Subsequently, the discs were transferred to 1000 µl of 0·5% saponin (mild detergent, Sigma Aldrich, Poznan, Poland) and vortexed vigorously to free the cells from the survival biofilm structure. Next, the obtained bacterial suspension was cultured on the appropriate stable medium. After 24 hours of incubation at 37°C, colony‐forming units were counted.
Results
The aim of this study was to develop quick and simple method of assessing antiseptics' efficacy against bacterial strains in the biofilm form. Therefore, because of its broad availability and low price the polystyrene well plate was chosen as a testing model. The first necessary step was to check whether the tested bacteria are actually able to form biofilm on the polystyrene within a standard incubation time of 24 hours. Therefore, the polystyrene discs were placed on the bottom of the plate wells and left them for the incubation time with the bacterial suspension. After 24 hours, the discs were checked for the presence of biofilm using electron microscopy (Figure 1 A,B). All tested strains were able to form biofilm on the polystyrene discs. However, the number of cells and the extracellular slime were different for different strains. The analysis of micrographs showed that the P. aeruginosa strains were generally able to produce more extracellular slime within 24 hours when compared with S. aureus strains. However, quantitative tests would need to be performed to prove it.
Figure 1.
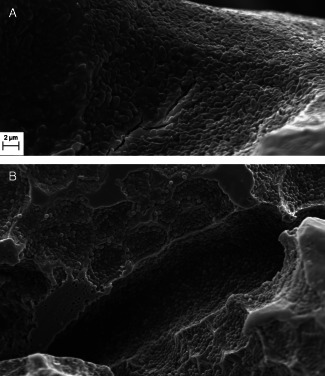
Staphylococcal and pseudomonal biofilm formed on the polystyrene disc. Scanning electron microscopy micrographs of Pseudomonas aeruginosa 15445 (A) and Staphylococcus aureus ATTC 5638 (B) biofilms formed on the surface of polystyrene discs. The adhered cocci and pseudomonal cells, as well as the extracellular layers visualised at the micrograph, confirm the ability of the investigated strains to form biofilm on the polystyrene within 24 hours. Scanning electron microscope ZEISS EVO MA 25.
The next step was a BOAT measurement as described in stages 1–7 of Materials and Methods, using three types of antiseptics whose active substances were povidone‐iodine, octenidine dihydrochloride and ethacridine lactate. The above‐mentioned antiseptic substances have different modes of action, covering generally the whole spectrum of mechanisms of action of these antimicrobials. Povidone‐iodine reacts with a key group of bacterial proteins, in particular with the free‐sulphur amino acids cysteine and methionine, nucleotides and fatty acids, which culminates in cell death 6. Octenidine dihydrochloride reacts with polysaccharides of bacterial compartments and enzymatic systems, which leads to the leakage of cytoplasmic components and impairment of cellular functions 8. Ethacridine lactate, in turn, reacts with bacterial DNA of the bacterial cell and inhibits the biosynthesis of nucleic acids 9. The results obtained using the BOAT assay are presented in Figure 2 and in Table 1.
Figure 2.
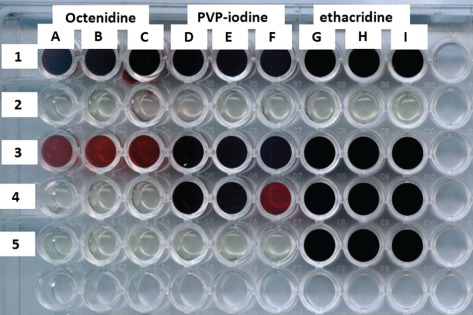
Sample biofilm‐oriented antiseptics test (BOAT). The bottoms of the indicated wells of the microtitre polystyrene assay plate are coated with bacterial biofilm. The dark red colour of the wells means that the bacteria in the biofilm form survived the treatment with the antiseptic within the contact time, whereas the lack of red colour is equivalent to a complete eradication of the bacteria forming biofilm in the well. Line 1 – positive control (biofilm untreated with antiseptic), confirming the ability of the tested strain 2034 to form biofilm at the bottom of the well; line 2 – negative control wells filled with the medium (no biofilm) confirming sterility of the assay performed; line 3: wells A, B, C – strain 2034 treated with octenidine for 1 minute; wells D, E, F – the same strain treated with PVP‐iodine; wells G, H, I – strain treated with ethacridine. Analogically, the biofilm from wells in lanes 4 and 5 was treated with the above‐mentioned antiseptics for 15 and 30 minutes, respectively.
Table 1.
The ability of the tested antiseptics to eradicate bacterial biofilm in the contact times*
| Pseudomonas aeruginosa | Staphylococcus aureus | |||||
|---|---|---|---|---|---|---|
| Contact time | Contact time | |||||
| 1 minute (%) | 15 minutes (%) | 30 minutes %) | 1 minute (%) | 15 minutes (%) | 30 minutes (%) | |
| PVP‐I | 0 | 33 | 66 | 100 | 100 | 100 |
| OCT | 0 | 46 | 100 | 100 | 100 | 100 |
| RIV | 0 | 0 | 0 | 60 | 70 | 100 |
PVP‐I, antiseptic containing povidone‐iodine; OCT, antiseptic containing octenidine dihydrochloride; RIV, antiseptic containing ethacridine lactate.
Fourteen strains of P. aeruginosa and S. aureus isolated from the infected leg ulcers were used for the experiment purposes. In addition, reference P. aeruginosa 14445 and S. aureus 5638 strains were used. The contact times were 1, 15 and 30 minutes. The percentage values indicate the number of strains in the biofilm form eradicated in the specified contact times by the antiseptics used.
The BOAT assay allows to estimate the efficacy of antiseptics in the real contact time (the time range used in this experiment was chosen according to the manufacturer's instructions) and in the real (working) solutions, which is problematic for planktonic‐oriented methods, where the working solution of the antiseptic has to be diluted with bacterial inoculum.
For P. aeruginosa strains, octenidine dihydrochloride was the most efficient in eradicating the biofilm from the polystyrene wells and PVP‐iodine proved to be more efficient than the ethacridine lactate‐based antiseptic (Table 1). However, it has to be stressed that all tested antiseptics were ineffective in the 1‐minute contact time against P. aeruginosa strains and complete eradication was achieved only when octenidine dihydrochloride was used for 30 minutes. In the case of PVP‐iodine, after the contact time of 30 minutes, 66% eradication was achieved. Interesting results were obtained for ethacridine lactate. Although the low efficacy of this antiseptic against coagulase‐negative staphylococci 10 and Klebsiella pneumoniae 11 was already demonstrated in our earlier works, BOAT has showed an extremely low suitability of ethacridine lactate against nosocomial strains of P. aeruginosa.
In the case of S. aureus strains, both octenidine dihydrochloride and PVP‐iodine were able to completely eradicate bacteria in the biofilm form, even in the shortest tested contact time of 1 minute. Again, the ethacridine‐based antiseptic proved to be the weakest among the tested antimicrobials, although satisfactory results were obtained after 15 and 30 minutes of contact time.
It has to be remembered that the basic BOAT version, hereinafter referred to as version A, allows to determine only if complete eradication of bacteria in the biofilm form took place. Therefore, another BOAT version (version B) that allows to estimate the ratio of eradication in terms of numbers of bacteria killed by the antiseptics was developed. The quantitative measurements of version ‘B’ were performed as described in ‘Quantitative Measurements of the Number of Colony‐Forming Units (BOAT Version B) to Prove the Results Obtained Using Qualitative Results (BOAT Version A)’. Among the tested strains, one strain of P. aeruginosa and one strain of S. aureus were selected on the basis of possible amount of data that could be obtained. The results obtained from the two versions (A and B) are presented in Table 2.
Table 2.
Comparison of two versions of BOAT techniques*
| Pseudomonas aeruginosa | Staphylococcus aureus | |||||
|---|---|---|---|---|---|---|
| BOAT version A | Contact time | Contact time | ||||
| 1 minute | 15 minutes | 30 minutes | 1 minute | 15 minutes | 30 minutes | |
| PVP‐I | − | − | + | + | + | + |
| OCT | − | + | + | + | + | + |
| RIV | − | − | − | − | + | + |
| P. aeruginosa | S. aureus | |||||
| BOAT version B | Number of cells/well survived treatment with antiseptic | Number of cells/well survived treatment with antiseptic | ||||
|---|---|---|---|---|---|---|
| 1 minute | 15 minutes | 30 minutes | 1 minute | 15 minutes | 30 minutes | |
| PVP‐I | 3·6(±0·3) × 104 | 2·1(±0·1) × 103 | 0 | 0 | 0 | 0 |
| OCT | 3·1(±0·2) × 104 | 0 | 0 | 0 | 0 | 0 |
| RIV | 4·3(±0·2) × 105 | 4·2 (±0·1) × 103 | 1·9(±0·5) × 102 | 5·3(±0·3) × 105 | 0 | 0 |
BOAT, biofilm‐oriented antiseptics test; PVP‐I, antiseptic containing povidone‐iodine; OCT, antiseptic containing octenidine dihydrochloride; RIV, antiseptic containing ethacridine lactate.
Version A of BOAT, less time‐ and labour‐consuming, allows to estimate if the specific antiseptic is efficient against the tested bacterial strain in the biofilm form in the specific contact time. In version A, the result is qualitative and ‘efficacy’ is understood as the ability to kill/inactivate all bacteria. Version B, more time‐ and labour‐consuming, allows to estimate the rate of bacterial eradication. Boat version A: ‘+’: complete eradication of bacteria in the biofilm form; ‘−’: incomplete eradication of bacteria in the biofilm form.
Discussion
The development of molecular and microscopic techniques observed in recent years has diametrically changed our understanding of infection mechanisms, moving stress to biofilm‐forming communities as the underlying cause of a majority of the infections. Therefore, BOAT assay was developed to help clinicists in choice of the most appropriate antiseptic in the treatment of local, biofilm‐related infections.
The most important question that has to be addressed is whether BOAT version A or version B would be more suitable for clinicists working in hospital microbiology laboratories. In our opinion, this is a rare example where a qualitative result (‘yes’ or ‘no’ answer) is more suitable than a quantitative result. To the best of our knowledge, there is presently no norm that would describe if a specific ratio of eradication of bacteria in the biofilm form can be treated as ‘effective’ or ‘ineffective’ eradication [for ‘planktonic‐oriented’ norms for antiseptics and disinfectants, such value is 99·9% of microorganisms killed 7]. Therefore, the simple result obtained using BOAT version A, indicating that all bacteria were eradicated, presents a higher value in terms of clinical use and might be used for choosing an appropriate antiseptic for local treatment. Hence, it is suggested that version A should be used in clinical routine, whereas the presented results concerning BOAT version B should be treated as supplementary data and may be applied for more basic science purposes.
References
- 1. Flemming H, Wingender J, Szewzyk U. Biofilm highlights. Springer Series on Biofilm. Vol. 5, 2011. ISBN 978‐3‐642‐19939‐4, Springer‐Verlag Berlin Heidelberg. [Google Scholar]
- 2. Shunmuganperumal T. Biofilm eradication and prevention. Hoboken: John Wiley & Sons, 2010. ISBN 978‐0‐470‐47996‐4. [Google Scholar]
- 3. Wolcott R, Dowd S, Kennedy J, Jones C. Biofilm‐based wound care. Adv Wound Care 2010;1:311–8. [Google Scholar]
- 4. Wolcott R, Rhoads D. Study of biofilm‐based wound management in subjects with critical limb ischaemia. J Wound Care 2008;17:145–55. [DOI] [PubMed] [Google Scholar]
- 5. Dowd E, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX Amplicon Pyrosequencing (bTEFAP). http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0003326 [accessed on 12 September 2008] [DOI] [PMC free article] [PubMed]
- 6. Kramer A, Müller O, Reichwagen G, Widulle S, Heldt H, Nürnberg P. Octenidine, chlorhexidine, iodine and iodophores. Stuttgart, New York: Georg Thieme, 2008. [Google Scholar]
- 7. European Norm . EN 1040 basic bactericidal activity of chemical disinfectants; European norms for disinfection testing. J Hosp Infect 2008;70(Suppl 1):8–10. [DOI] [PubMed] [Google Scholar]
- 8. Hubner NO, Siebert J, Kramer A. Octenidine dihydrochloride, a modern antiseptic of skin, mucous membranes and wounds. Skin Pharmacol Physiol 2010;23:244–58. [DOI] [PubMed] [Google Scholar]
- 9. Wainwright M. Acridine—a neglected antibacterial chromophore. J Antimicrob Chemother 2001;47:1–13. [DOI] [PubMed] [Google Scholar]
- 10. Bartoszewicz M, Junka A, Smutnicka D. Sensitivity of nosocomial Klebsiella pneumoniae strains to antiseptics used in wound treatment. Forum Zakaz 2011;4:121–7 article in Polish. [Google Scholar]
- 11. Bartoszewicz M, Junka A, Smutnicka D. Efficacy of topical antiseptics against nosocomial Klebsiella pneumoniae strains. Forum Zakaz 2011;4:121–7. [Google Scholar]


