Abstract
Pulsed radio frequency energy (PRFE) has successfully been used to treat diabetic and venous stasis ulcers. In this case report, a lightweight wearable form of a PFRE device was evaluated and used to treat three diabetic foot ulcers and one venous stasis ulcer. The ulcers were present on the four patients for more than 3 months and had failed to heal after conventional treatment. A lightweight battery‐powered, wearable form of PRFE device was introduced as a treatment and used 6–8 hours per day for a period of 6 weeks. All patients after 1 week of therapy showed improvement and wound size was seen to decrease. Patient 1 had a venous stasis ulcer, and reported significant pain relief after 2 weeks treatment. Patients 2 and 3 achieved complete healing after 3 weeks treatment, and patients 1 and 4 had a 95% and 88% reduction in wound size, respectively, after the 6‐week study period. Both these patients continued to complete healing using the PRFE device after the 6‐week study period. PRFE treatment delivered in the form of a wearable lightweight patch appears to offer promise in the treatment of recalcitrant chronic wounds.
Keywords: Chronic, Device, Healing, PRFE, Wounds
INTRODUCTION
Diabetic foot ulcers are the most common chronic wounds in western industrialised countries. Of the millions who have diabetes mellitus, 15% will suffer of foot ulceration which often leads to amputation (100, 000 per annum in the US alone). The economic burden of treating diabetes as its associated complications is extreme (1) and will likely increase as the rate of diabetes continues to rise. Statistics from the American Diabetes Association show the prevalence of diabetes at 25·8 million children and adults, or 8·3% of the US population. Venous stasis ulcers are a major cause of chronic wounds, and are typically associated with significant pain. Venous stasis ulcers are common in patients who have a history of leg swelling, varicose veins, or a history of blood clots in either the superficial or the deep veins of the legs. Venous ulcers is the most common aetiology of lower extremity ulceration, affecting approximately 1% of the US population (2).
The healing of diabetic foot ulcers, is necessary for the prevention of amputation and a number of advanced technologies have been introduced to achieve higher success in amputation prevention and limb preservation (3). Electrotherapy in the form of pulsed radio frequency electromagnetic (PRFE) energy has recently received a new focus, with a number of case reports showing promising results in the healing of chronic wounds 4, 5, 6, 7, 8. A retrospective study on the Regenesis Wound Healing Registry (Regenesis Biomedical, Scottsdale, AZ) has indicated that PRFE therapy holds promise to be an effective treatment for chronic wounds (9). Regensis Biomedical's Provant System is a suitcase‐sized device that emits non‐ionising, radio frequency energy at 27·12 MHz. There is a growing list of clinical studies that have shown the safety and efficacy of PRFE as a therapy, as has been recently reviewed by Guo et al. (10). However, there are still major limitations to PRFE devices, as treatment regimens require 2 × 30 minute treatments per day, making it impractical for most ambulatory patients, restricting its use to severe chronic wounds.
In this case report, we show the application of a wearable battery‐powered form of PRFE device for the treatment of recalcitrant wounds. The lower energy levels emitted by this form of PRFE device are compensated by extended use times. The PRFE device used in this case study was ActiPatch™ (BioElectronics Corporation, Frederick, MD) which delivers PRFE at a carrier frequency of 27·12 MHz and a pulse rate of 1000 Hz.
MATERIALS AND METHODS
At the Temple University Foot and Ankle Institute, four adult African‐American diabetic males between the ages of 40 and 75 with ulcers present for longer than 3 months were admitted into the pilot study. Three patients had diabetic neuropathic ulcers and one had a venous stasis ulcer. All the diabetic ulcer patients had at least one palpable pedal pulse and an ulcer of Wagner Grade II or higher.
All ulcers had previously been treated with a variety of methods, without appreciable healing, and are described for each patient. Patient 1 was 72 years old with type II diabetes that had a venous stasis ulcer that had undergone multilayer compression therapy for 4 weeks without any appreciable healing. Significant pain was experienced by this patient who was assessed by a 0–10 point visual analogue scale (VAS). Patient 2 was 42 years old with type II diabetes and an actively working truck driver. Previous failed treatment included wound debridement, use of Promogran matrix and dry sterile dressing. Once the PRFE device was added to the regimen, Promogran was discontinued. Patient 3 was a 62‐year‐old patient with insulin‐controlled diabetes that had not responded to debridement and application of triple antibiotic ointment with offloading. Once the PRFE device was added, triple antibiotic was discontinued. Patient 4 was a 74‐year‐old patient with insulin‐controlled diabetes presented with a right heel decubitus ulcer that resulted following hospitalisation for prostate surgery. He had already had a below knee amputation on the left side. The right heel wound was granular and non‐infected. Offloading with a protective boot and wound care consisting of debridement, Promogran matrix and dry sterile dressings were done prior to the PRFE device use. Once the PRFE device was used, weekly debridement and offloading was maintained. After informed consent, patients adopted a protocol that used the PRFE device for 6–8 hours per day. The patients with diabetic ulcers had their wounds covered with moist saline gauze, ActiPatch™, and a dry sterile dressing. When the ActiPatch™ PRFE device was not in use, the ulcer was covered with moist saline gauze and dry sterile dressing. Compression therapy was continued with patient 1 along with the PRFE device for 6–8 hours per day. Patients kept a journal of their PFRE device use and brought the log in during their weekly visits. Weekly visits consisted of sharp debridement and surgical scrub, for the diabetic ulcer patients, followed by measurement and photographic documentation. Wounds were evaluated for any signs of infection and new changes such as increased depth or drainage. The PFRE device was also evaluated for proper functioning at each visit. Patients were educated on their daily wound dressing changes. The wounds were evaluated once weekly for a total of 6 weeks.
RESULTS
The patients tolerated the PRFE therapy well and reported no negative side effects. The diabetic ulcers still needed to be sharply debrided on a weekly basis, but patients were pleased with the therapy and its ease of use at home. Table 1 contains the patient age, wound location and wound measurements in centimeters at the start of the treatment (week 0) and for the following 6 weeks of treatment on a weekly basis. Starting at week 1, all patients were seen to have a decrease in wound size. The ulcers had a steady decrease in side to side closure and in visible periwound oedema. Patients 2 and 3 had complete healing of their diabetic ulcers after 3 weeks of treatment.
Table 1.
The patient age, ulcer location and ulcer size data (in centimeters) of each patient at week 0 (start of treatment) and for each week of the 6‐week study period
| Patient | Age | Location | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | Right leg | 4 × 2·5 | 4 × 2·3 | 4 × 2 | 3 × 1·5 | 2 × 1·5 | 1 × 0·7 | 0·7 × 0·5 |
| 2 | 60 | Right foot | 0·5 × 0·5 | 0·3 × 0·3 | 0·2 × 0·1 | ulcer healed | |||
| 3 | 43 | Left heel | 4 × 1 | 2 × 0·5 | 1 × 0·3 | ulcer healed | |||
| 4 | 74 | Right heel | 2·5 × 1·75 | 2 × 2 | 2 × 1·5 | 1·7 × 0·7 | 1 × 1 | 1 × 0·5 | 1 × 0·5 |
Patient 1 had a venous stasis ulcer, as shown in Figure 1, which caused the patient significant pain. After 2 weeks of PRFE therapy the patient reported significant pain relief. The ulcer of patient 1 decreased in size from 4 × 2·5 cm to 0·7 × 0·5 cm at the end of the 6‐week study period, a decrease of approximately 95% of the wound area. The venous stasis ulcer continued to complete healing after the study period with continued use of the PRFE therapy device.
Figure 1.
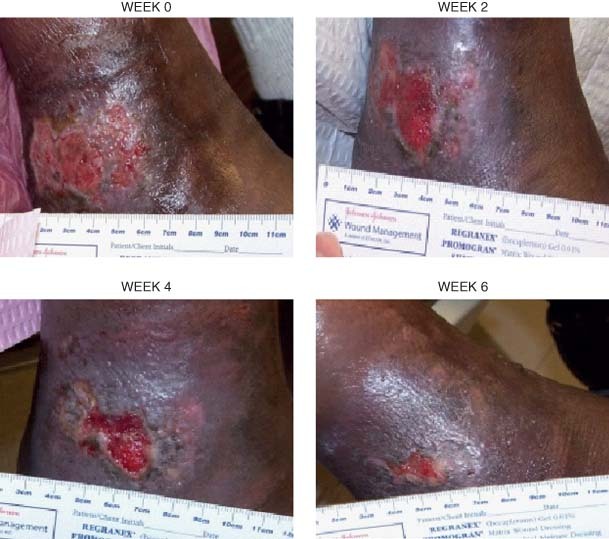
The venous stasis ulcer of patient 1 is shown at weeks 0, 2, 4 and 6 of PRFE treatment. Significant pain relief was reported by the patient after 2 weeks treatment. The ulcer had decreased in size from 4·0 × 2·5 cm to 0·7 × 0·5 cm after 6 weeks PRFE treatment. The ulcer continued onto healing using the PRFE therapy.
The right foot ulcer of patient 2 is shown in Figure 2 and the left heel ulcer of patient 3 is shown in Figure 3. Both ulcers of patients 2 and 3 improved rapidly with PRFE treatment, recovering up to 50% of the wound area after 1 week of PRFE treatment. The ulcers progressed to complete healing after 3 weeks of PRFE treatment.
Figure 2.
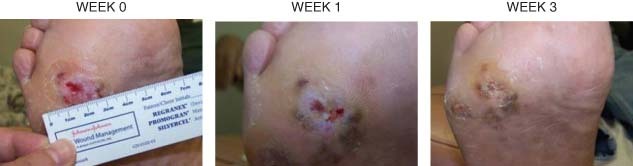
Patient 2 had a 0·5 × 0·5 cm diabetic ulcer at the beginning of PRFE treatment, which healed after 3 weeks PRFE therapy. The ulcer at weeks 0, 1 and 3 is shown.
Figure 3.
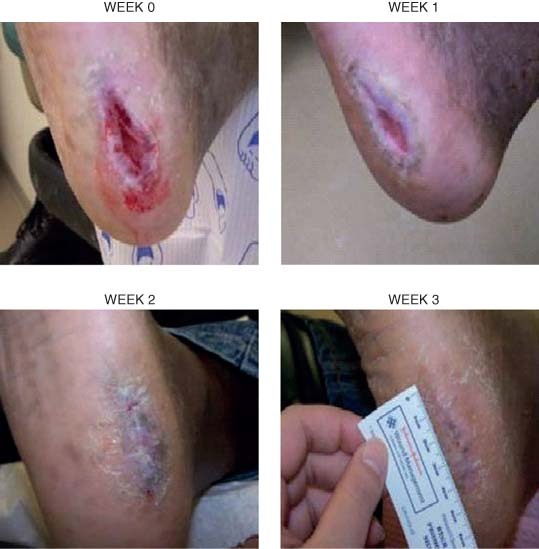
Patient 3 had a 4·0 × 1 cm diabetic ulcer on the left heel. After 3 weeks of PRFE treatment the ulcer had completely healed. The ulcer is shown at weeks 0, 1, 2 and 3.
The diabetic ulcer of patient 4 had a wound size at the beginning of treatment of 2·5 × 1·75 cm, by week 4 this had decreased to 1 × 1 cm, approximately a 73% reduction in size and by week 6 the wound area decreased to 1 × 0·5 cm, a reduction of 88% in size. Wound area reduction at weeks 1 and 4 is a strong indicator of complete healing (11). Patient 4 had significant reduction in wound size at week 6 and continued on to healing after the study period, using the PRFE treatment.
DISCUSSION
The mechanism by which PRFE promotes the healing of chronic wounds is not fully understood. But studies on cells and animals have given insight into the effects of PRFE therapy. Upregulation of gene families involved in tissue repair in co‐cultures of human dermal fibroblasts and epidermal keratinocytes treated with PRFE has been shown (12). These included metalloproteinase and tissue inhibitor of metalloproteinase, interleukin‐related genes, interferon‐related genes and tumour necrosis factor‐related genes. Cell studies have demonstrated a upregulation of fibroblast growth factor‐2 (FGF‐2) after PRFE exposure, an important molecule in wound healing for promoting endothelial cell proliferation, angiogenesis and granulation tissue formation. PRFE therapy given to animal wound models of diabetes have reported to an upregulation of FGF‐2 (13), increased wound healing and wound tensile strength compared to sham control animals 14, 15.
A portable wearable PRFE device was first used in 1982 for the treatment of postoperative wounds following blepharoplasty (16). A study by Stiller et al., using a wearable form of PFRE device evaluated its clinical efficacy and safety in a placebo‐controlled multicenter trial. Significant decreases in wound depth and pain intensity favouring the active group were seen, wounds after 8 weeks of treatment in the active group had a 47·7% decrease in wound surface area versus a 42·3% increase for placebo (17). The device used in this study weighed 505 g and was used 3 hours per day. More recently clinical trials on the postoperative recovery after breast augmentation surgery (18) and breast reduction surgery (19) have clearly demonstrated the control of postoperative pain with newer versions of wearable, portable PRFE devices.
In this pilot study presented here, a light weight wearable form of PRFE, ActiPatch™, was used to facilitate the wound healing process in both diabetic and venous stasis lower extremity ulcers. The PRFE device used in this case study was in the form a patch and was easy to use from both the physician's and patient's standpoint. Since the completion of this study, ActiPatch™ has been refined and updated, and now consists of a small control module and a 12 cm or 8 cm antenna and weighs approximately 8 g. The reconfigured device is now used as a 24‐hour continuous PRFE therapy with the same carrier frequency of 27·12 MHz and 1000 Hz pulse rate. Figure 4 shows the latest version of the PRFE device and Figure 5 shows the application of the PRFE device on a patient with a venous stasis ulcer; prior to PRFE therapy this patient was considered for amputation, however, the ulcer healed within 8 weeks and the patient avoided amputation (unpublished data).
Figure 4.

The latest version of the PRFE device is shown, consisting of a small control module and a wire antenna, either 12 cm or 8 cm. The PRFE device is used 24 hours per day, is lightweight weighing approximately 8 g and provides treatment continuously for 7 days.
Figure 5.
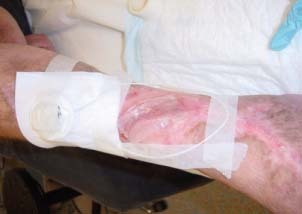
A version of the PRFE device is shown in use on a patient with a venous stasis ulcer.
The results from this pilot study suggest that lightweight wearable PRFE devices maybe an effective adjunct therapy for recalcitrant wounds promoting healing and reducing pain. The ease of use, low cost and compatibility with current conventional therapy also suggest this form of wearable PRFE device could be widely applied as a first choice therapy, although further studies are required to determine their true value.
REFERENCES
- 1. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010;100:335–41. [DOI] [PubMed] [Google Scholar]
- 2. Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician 2010;81:989–96. [PubMed] [Google Scholar]
- 3. Wu SC, Marston W, Armstrong DG. Wound care: the role of advanced wound healing technologies. J Vasc Surg 2010;52 Suppl 3:59S–66S. [DOI] [PubMed] [Google Scholar]
- 4. Larsen JA, Overstreet J. Pulsed radio frequency energy in the treatment of complex diabetic foot wounds: two cases. J Wound Ostomy Continence Nurs 2008;35:523–7. [DOI] [PubMed] [Google Scholar]
- 5. Porreca EG, Giordano‐Jablon GM. Treatment of severe (Stage III and IV) chronic pressure ulcers using pulsed radio frequency energy in a quadriplegic patient. Eplasty 2008;8:e49. [PMC free article] [PubMed] [Google Scholar]
- 6. Fletcher S. Successful treatment of venous stasis ulcers with combination compression therapy and pulsed radio frequency energy in a patient scheduled for amputation. J Wound Ostomy Continence Nurs 2011;38:91–4. [DOI] [PubMed] [Google Scholar]
- 7. Frykberg R, Martin E, Tallis A, Tierney E. A case history of multimodal therapy in healing a complicated diabetic foot wound: negative pressure, dermal replacement and pulsed radio frequency energy therapies. Int Wound J 2011;8:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maier M. Pulsed radio frequency energy in the treatment of painful chronic cutaneous wounds: a report of two cases. Pain Med 2011;12:829–32. [DOI] [PubMed] [Google Scholar]
- 9. Frykberg RG, Driver VR, Lavery LA, Armstrong DG, Isenberg RA. The use of pulsed radio frequency energy therapy in treating lower extremity wounds: results of a retrospective study of a wound registry. Ostomy Wound Manage 2011;57:22–9. [PubMed] [Google Scholar]
- 10. Guo L, Kubat NJ, Isenberg RA. Pulsed radio frequency energy (PRFE) use in human medical applications. Electromagn Biol Med 2011; 30:21–45. [DOI] [PubMed] [Google Scholar]
- 11. Lavery LA, Barnes SA, Keith MS, Seaman JW Jr, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care 2008;31:26–9. [DOI] [PubMed] [Google Scholar]
- 12. Moffett J, Griffin N, Ritz M, George F. Pulsed radio frequency energy field treatment of cells in culture results in increased expression of genes involved in the inflammation phase of lower extremity diabetic wound healing. J Diabet Foot Complicat 2010;2:57–64. [Google Scholar]
- 13. Callaghan MJ, Chang EI, Seiser N, Aarabi S, Ghali S, Kinnucan ER, Simon BJ, Gurtner GC. Pulsed electromagnetic fields accelerate normal and diabetic wound healing by increasing endogenous FGF‐2 release. Plast Reconstr Surg 2008;121:130–41. [DOI] [PubMed] [Google Scholar]
- 14. Goudarzi I, Hajizadeh S, Salmani ME, Abrari K. Pulsed electromagnetic fields accelerate wound healing in the skin of diabetic rats. Bioelectromagnetics 2010;31:318–23. [DOI] [PubMed] [Google Scholar]
- 15. Li Q, Kao H, Matros E, Peng C, Murphy GF, Guo L. Pulsed radio frequency energy (PRFE) accelerates wound healing in diabetic mice. Plast Reconstr Surg 2011;127:2255–62. [DOI] [PubMed] [Google Scholar]
- 16. Nicolle FV, Bentall RM. Use of radio‐frequency pulsed energy in the control of postoperative reaction in blepharoplasty. Aesthetic Plast Surg 1982;6:169–71. [DOI] [PubMed] [Google Scholar]
- 17. Stiller MJ, Pak GH, Shupack JL, Thaler S, Kenny C, Jondreau L. A portable pulsed electromagnetic field (PEMF) device to enhance healing of recalcitrant venous ulcers: a double‐blind, placebo‐controlled clinical trial. Br J Dermatol 1992;127:147–54. [DOI] [PubMed] [Google Scholar]
- 18. Heden P, Pilla AA. Effects of pulsed electromagnetic fields on postoperative pain: a double‐blind randomized pilot study in breast augmentation patients. Aesthetic Plast Surg 2008;32:660–6. [DOI] [PubMed] [Google Scholar]
- 19. Rohde C, Chiang A, Adipoju O, Casper D, Pilla AA. Effects of pulsed electromagnetic fields on interleukin‐1 beta and postoperative pain: a double‐blind, placebo‐controlled, pilot study in breast reduction patients. Plast Reconstr Surg 2010;125:1620–9. [DOI] [PubMed] [Google Scholar]


