Abstract
Objective
Increasing reports suggest a role for immunological mechanisms in febrile infection‐related epilepsy syndrome (FIRES). The objective of this study was to elucidate the efficacy and safety of intrathecal dexamethasone therapy (IT‐DEX).
Methods
We assessed six pediatric patients with FIRES who were administered add‐on IT‐DEX in the acute (n = 5) and chronic (n = 1) phases. We evaluated clinical courses and prognosis. We measured cytokines/chemokines in cerebrospinal fluid (CSF) from FIRES patients at several points, including pre‐ and post‐IT‐DEX, and compared them with control patients with chronic epilepsy (n = 12, for cytokines/chemokines) or with noninflammatory neurological disease (NIND, n = 13, for neopterin).
Results
Anesthesia was weaned after a median of 5.5 days from IT‐DEX initiation (n = 6). There was a positive correlation between the duration from the disease onset to the introduction of IT‐DEX and the length of ICU stay and the duration of mechanical ventilation. No patient experienced severe adverse events. Seizure spreading and background activities on electroencephalography were improved after IT‐DEX in all patients. The levels of CXCL10, CXCL9, IFN‐γ, and neopterin at pre‐IT‐DEX were significantly elevated compared to levels in epilepsy controls, and CXCL10 and neopterin were significantly decreased post‐IT‐DEX, but were still higher compared to patients with chronic epilepsy. IL‐6, IL‐8, and IL‐1β were significantly elevated before IT‐DEX compared to epilepsy controls, though there was no significant decrease post‐treatment.
Interpretation
IT‐DEX represents a therapeutic option for patients with FIRES that could shorten the duration of the critical stage of the disease. The effect of IT‐DEX on FIRES might include cytokine‐independent mechanisms.
Introduction
Febrile infection‐related epilepsy syndrome (FIRES) is an epileptic encephalopathy of unknown etiology affecting previously healthy children following febrile illness. 1 FIRES has been defined as a subcategory of new‐onset refractory status epilepticus (NORSE) and is almost identical to previous nomenclatures including acute encephalitis with refractory, repetitive partial seizures (AERRPS), or devastating epileptic encephalopathy in school‐aged children (DESC). 2 , 3 , 4 Although immunological mechanisms are implicated in this condition, the exact underlying pathogenic process has not been elucidated. Conventional immuno‐modulatory therapies such as corticosteroid therapy or IV immunoglobulin (IVIg) therapy sometimes lead to positive responses but are not sufficiently efficacious in most cases. 5 , 6 Ketogenic diet therapy seems to be a promising candidate therapy, but is not always effective. 7 , 8 Patients with FIRES often need prolonged barbiturate‐induced comas due to the extraordinarily high epileptic activity, sometimes developing life‐threatening complications during prolonged intensive care unit (ICU) stays. 9 According to multiple follow‐up studies, outcomes in epilepsy and cognitive function remain unfavorable. 10 , 11
Early diagnosis and intervention seem to be key for a better prognosis. Although a specific biomarker is lacking, selective upregulation of cerebrospinal fluid (CSF) proinflammatory cytokines is a hallmark of FIRES. 12 This observation led us to speculate that local inflammation in the central nervous system plays significant roles in the development of FIRES and thus targeting neuroinflammation might be a promising therapeutic approach.
Here, we used intrathecal injection of dexamethasone (IT‐DEX) in six patients with FIRES as a new approach. We also show serial data on neopterin and cytokines/chemokine concentrations in CSF from these patients.
Materials and Methods
Patients
Six patients with a diagnosis of FIRES who were referred to Osaka City General Hospital and received IT‐DEX therapy from 2014 to 2018 were enrolled. For diagnosis of FIRES, the previous criteria were used with slight modifications 2 , 13 : (1) Acute onset of super‐refractory status epilepticus (SRSE) in the absence of underlying developmental delay or prior unprovoked seizures (The patients usually show focal onset seizures); (2) Antecedent febrile illness, which occurs 2–10 days before the onset of neurological symptoms; (3) Absence of a specific brain structural abnormality, infection, toxic insult, or metabolic cause that could explain the SRSE. Eligible patients in the current study fulfilled the consensus diagnostic criteria of FIRES published in 2018. 11
IT‐DEX was added after a minimum of one course of conventional immunotherapy (IVMP or IVIg) had been completed. Information about clinical manifestation, continuous electroencephalography (EEG) findings, magnetic resonance imaging (MRI) findings, treatment, adverse events, and follow‐up clinical outcomes were collected. We summarized anti‐seizure treatment and immunotherapies administered during the ICU stay, continuous EEG monitoring data including ictal and interictal conditions at pre‐ and post‐IT‐DEX, epilepsy outcome, and neuropsychological evaluation at hospital discharge. Series data of cytokines/chemokines and neopterin in CSF were also analyzed. The day on which CSF was examined for pre‐IT‐DEX ranged from 7 to 56 (median 14.5) days from disease onset, and the post‐IT‐DEX assessment period ranged from 19 to 75 (median 28.5) days.
IT‐DEX treatment regimen
IT‐DEX was initiated at a dosage of 0.15–0.25 mg/kg/day. The treatment was repeated four times and added a few times depending on the particular situation of the patients. The dosing interval was 1–6 days.
Immunological data
Cytokine/chemokine assays
CSF levels of cytokines and chemokines (CXCL9, CXCL10, IFN‐γ, IL‐1β, IL‐6, and IL‐8) were measured using a Bio‐Plex Suspension Array System (Bio‐Rad, Hercules, CA, USA) according to the manufacturer’s protocols. For comparisons with control subjects, data from 12 patients with epilepsy were obtained from Tokyo Metropolitan Institute of Medical Science. Data below detectable levels were set at the lowest value of the dynamic range of detection.
Neopterin assays
We performed CSF neopterin analyses using high‐performance liquid chromatography (HPLC) with fluorometric detection. 14 Data were compared to those of 13 patients with noninflammatory neurological disorders (NIND) recruited from Osaka City General Hospital.
EEG studies
EEG recordings on the examination day are shown in Table 1 and were reviewed by more than two board‐certified pediatric neurologists and board‐certified pediatric epileptologists. Electrodes were placed according to the international 10–20 system. We evaluated each 12 hours of continuous EEG recording at the closest timing to IT‐DEX initiation and completion as pre‐IT‐DEX and post‐IT‐DEX, respectively. We evaluated predominant background activity, ictal EEG patterns, and frequency of electrographic seizures based on continuous EEG. When the number of seizures post‐IT‐DEX decreased to more than half the level of pre‐IT‐DEX, we defined it as a seizure decrease. Ictal EEG patterns were divided into four types: focal or regional, hemispheric, shifting, and generalized pattern. 15 , 16 Shifting seizure was defined as a seizure which alternated between hemispheres (bilateral independent) or a seizure which focus migrated after a focal onset to the contralateral hemisphere while still continuing in the hemisphere of onset.
Table 1.
Clinical features of the six patients and therapy regimen of IT‐DEX.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Age at onset/Sex | 4y/M | 8y/M | 6y/F | 7y/M | 6y/M | 5y/M |
| Duration from fever to seizure onset(days) | 4 | 4 | 5 | 6 | 3 | 6 |
| The maximum dose of barbiturate(mg/kg/hr) | 7 | 10 | 7 | 5 | 12 | 9 |
| Head MRI findings (the day after onset) | (1) DWI HI at Lt. occipital lobe and Lt. HC, (3) DWI HI at bil. HC | (1) Normal, (4) HI at HC, (22) HI and atrophy at bil. HC | (2)DWI HI in putamen and caudate nucleus | (27) HI at HC | (1)Normal,(31)DWI HI at BG | (83) Cerebral atrophy, HI at cortex, and pons |
| Initiation day of IT‐DEX | 9 | 10 | 10 | 20 | 20 | 59 |
| Frequency of use IT‐DEX | 6 | 8 | 7 | 4 | 5 | 7 |
| IT‐DEX dose (mg/kg/dose) | 0.22 | 0.17 | 0.17 | 0.18 | 0.15 | 0.18 |
| Anti‐seizure drugs prior to IT‐DEX administration | DZP, MDL, fPHT, TPL | MDL, TPL, TPM, LEV | DZP, MDL, fPHT, TPL, LEV, PB, KM | MDL, fPHT, TPL, LEV, ZNS, CLB, TPL, TPM, PB | MDL, TPL, TPM, LEV, fPHT, PB, KM | MDL, fPHT, PB, LEV, CLB, KBr, Thiamylal, TPL,TPM, KM, KD |
| Immunomudulating drugs prior to IT‐DEX administration(the number of times) | IVIG(1), IVMP(1) | IVIG(1), IVMP(2) | IVIG(1), IVMP(1) | IVIG(1), IVMP(1) | IVIG(2), IVMP(3) | IVIG(2), IVMP(2) |
| Drugs with IT‐DEX administration | PB, LEV, TPM, TPL | PB, LEV, TPM, TPL | TPL, KM, LEV, PB, PER | PB, LEV, TPM, TPL | TPL, MDL | MDL, TPM, VPA, PER |
Abbreviations: BG, basal ganglia; Bil.:bilateral; CLB, clobazam; DWI, diffusion‐weighted images; DZP, diazepam; fPHT, fosphenytoin; HC :hippocampus; HI, high intensity; IVIg, intravenous immunoglobulin; IVMP, intravenous methyl prednisolone; KBr, potassium bromide; KD, ketogenic diet; KM, ketamine; LEV, levetiracetam; lt, left; MDL, midazolam; MRI, magnetic resonance image; PB, phenobarbital; PER, perampanel; rt, right; TPL, thiopental; TPM, topiramate; VPA, sodium valproate.
Outcome assessment
The changes in EEG findings including seizure frequency, seizure spreading, and background activity between pre‐ and post‐IT‐DEX, duration from IT‐DEX initiation to thiopental discontinuance, duration of mechanical ventilation, and adverse events during IT‐DEX therapy were evaluated. Cognitive outcomes represented by the pediatric cerebral performance category scale (PCPC), and seizure frequency at discharge from the hospital were assessed as the secondary outcome.
Statistical analysis
Statistical analyses for comparisons between two groups were performed using a Mann–Whitney U test. A Wilcoxon signed‐rank test was used for comparisons between pre‐ and post‐IT‐DEX data. Correlations between neopterin and cytokine/chemokine concentrations in CSF were assessed using a Spearman's rank correlation coefficient. These analyses were conducted using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA). Differences with P < 0.05 were considered statistically significant.
Standard protocol approval, registrations, and patient consents
This retrospective study protocol was approved by the Ethics Committee of Osaka City General Hospital. Written consent for the study was obtained from the patients’ parents.
Results
The clinical features and pretreatment regimens of the six patients included in this study are shown in Table 1. One patient was referred to our facility during the chronic phase of illness at 2 months after onset. Figure 1 shows the treatment course of the six patients with FIRES during intensive care (Fig. 1). Four of the six patients needed sustained thiopental to control seizures at the time of initiation of IT‐DEX treatment. The rest of the two patients had shown SE persisting despite burst‐suppression coma (BSC). Six patients were withdrawn from anesthetic agents and were placed under intravenous infusion of AED and/or enteral AED, after a median of 5.5 days from the initiation of IT‐DEX.
Figure 1.
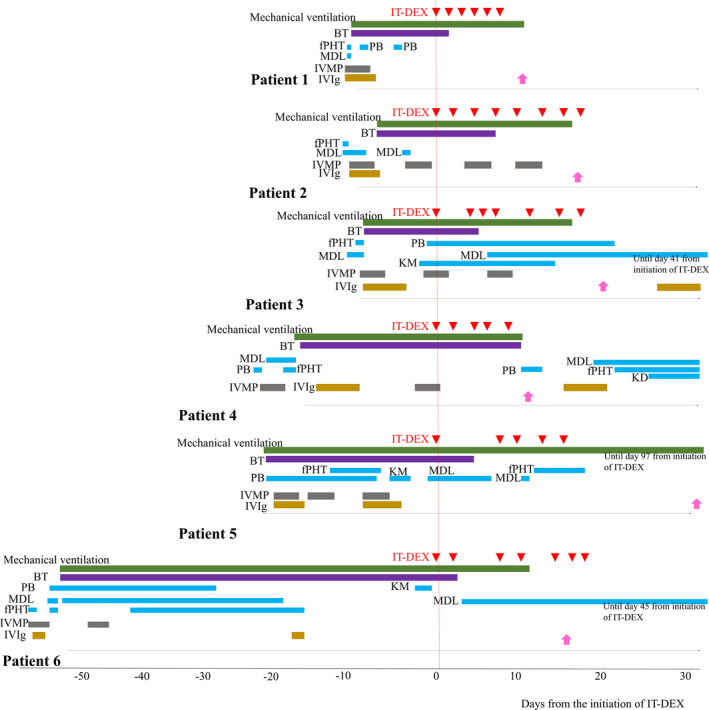
The treatment course of the six patients with FIRES during intensive care. Intravenous antiepileptic drugs, immunotherapies, and ketogenic diet therapy are shown. Pink arrows represent discharge from ICU. AED, antiepileptic drugs; BT, barbiturate therapy; fPHT, fosphenytoin; KM, ketamine; MDL, midazolam; KD, ketogenic diet therapy; IVMP, intravenous methylprednisolone; IVIg, intravenous immunoglobulin.
EEG findings at pre‐ and post‐IT‐DEX and clinical outcomes following IT‐DEX are shown in Table 2. Seizure frequency decreased after IT‐DEX in three patients (50%). Seizure spreading was localized and background activities improved in all patients.
Table 2.
EEG findings at pre‐ and post‐IT‐DEX.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Pre‐IT‐DEX | ||||||
| Day of examination | 8 | 9 | 10 | 20 | 19 | 59 |
| Intravenous AED | TPL | TPL | KM, TPL, PB | TPL | TPL, fPHT | TPL, MDL |
| Enteral AED | PB, LEV, TPM | LEV | LEV | LEV, TPM, PB | PB, LEV | TPM |
| Predominant BGA | Diffuse high voltage slow activity | Burst suppression | Burst suppression | Diffuse high voltage slow activity | Burst suppression | Delta‐theta wave dominant |
| Main seizure pattern | Hemispheric | Shifting | Hemispheric | Shifting | Generalized | Shifting |
| Post‐IT‐DEX | ||||||
| Day of examination | 19 | 35 | 29 | 38 | 32 | 78 |
| Intravenous AED | None | None | MDL, PB | MDL | fPHT | MDL |
| Enteral AED | PB,LEV,TPM | LEV,PB,TPM | PER,CZP,VPA | LEV,TPM, PB | None | PER,VPA,TPM |
| Predominant BGA | Delta‐theta wave | Theta wave | Alpha wave | Theta wave | Delta‐theta wave | Alpha wave |
| Main seizure pattern | Focal or regional | Focal or regional | Focal or regional | Focal or regional | Hemispheric | Focal or regional |
| Seizure frequency | No change | Decrease | Decrease | No change | No change | Decrease |
| Seizure spreading | Localized | Localized | Localized | Localized | Localized | Localized |
Abbreviations: AED, antiepileptic drug; BGA, back ground activity; CZP, clonazepam; fPHT, fosphenytoin; KM, ketamine; LEV, levetiracetam; MDL, midazolam; PB, phenobarbital; PER, perampanel; TPL, thiopental; TPM, topiramate; VPA, sodium valproate.
Five patients to whom IT‐DEX was administered during the acute phase were taken off thiopental after a median of 5.0 days from the initiation of IT‐DEX (Table 3). In Patient 6, thiopental was discontinued after 3 days from the induction of IT‐DEX in the chronic phase of illness. The dose of midazolam required after the completion of thiopental in Patients 3, 4, and 6 was low; less than the anesthesia dose. The median period of mechanical ventilation in the six patients was 26.5 days (mean 46, range 17–117 days). There was a positive correlation between the length of stay in the ICU and the duration from the disease onset to the introduction of IT‐DEX (rs = 0.85, P = 0.03) and the duration of mechanical ventilation (rs = 0.88, P = 0.03). (Supplementary Fig. S1).
Table 3.
Clinical course and outcome in six patients with FIRES.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Duration between IT‐DEX initiation and TPL withdrawal (days) | 3 | 5 | 5 | 10 | 6 | 3 |
| The period of mechanical ventilation | 17 | 22 | 24 | 29 | 117 | 65 |
| The day of release from ICU | 20 | 23 | 28 | 31 | 71 | 74 |
| Adverse effect with IT‐DEX | None | None | None | None | None | None |
| Outcome at discharge from hospital | ||||||
| The day of discharge from the hospital | 76 | 87 | 81 | 149 | 393 | 94 |
| Seizure frequency | monthly | weekly | monthly | weekly | weekly | annually |
| PCPC | 3 | 3 | 2 | 3 | 4 | 3 |
| Outcome at the last follow up | ||||||
| Seizure frequency | monthly | weekly | none | monthly | weekly | none |
| Duration of observation (months) | 70 | 62 | 18 | 53 | 76 | 17 |
Abbreviations: ICU, intensive care unit; PCPC, pediatric cerebral performance category scale.
Two patients out of the six showed a good epilepsy outcome at the last follow‐up. In Patient 3 and Patient 6, epileptic seizures were not detected after day 50 or day 103, respectively, although inter‐ictal EEG examination of both these patients demonstrated that epileptic discharges were dominant at the temporal and frontal lobe at the last follow‐up. All patients except one showed more than PCPC 3 at hospital discharge.
Figure 2 shows the cytokine/chemokine and neopterin data for the CSF (Fig. 2). The neopterin concentrations in the CSF correlated with CXCL9 and CXCL10 concentrations (rs = 0.74, P < 0.001 for CXCL9, rs = 0.77, P < 0.001 for CXCL10). CSF concentrations of CXCL10 and/or neopterin were determined within 2 weeks from disease onset in all six patients, and the values included the peak value of the series data in each patient with the exception of Patient 6. In Patient 6, CXCL10 peaked during IT‐DEX administration during the chronic phase, but there was no sharp fluctuation in concentrations.
Figure 2.
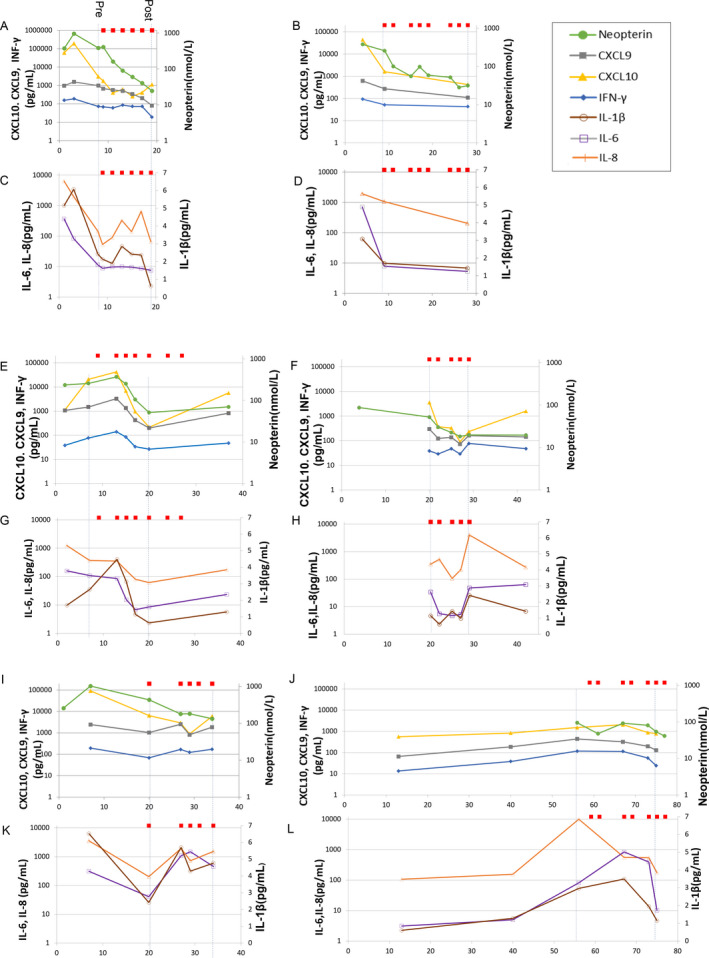
CSF concentrations of cytokines, chemokines, and neopterin in the six patients with FIRES. Red squares show the administration of IT‐DEX. The x‐axis represents days from the onset of disease. The y‐axis represents CXCL10, CXCL9, IFN‐γ, neopterin, IL‐6, and IL‐8 concentrations presented in log scale (A–L). Patient 1 (A, C); Patient 2 (B, D); Patient 3 (E, G); Patient 4 (F, H); Patient 5 (I, K); and Patient 6 (J, L).
The concentrations of CXCL10, CXCL9, IFN‐γ, and neopterin at pre‐IT‐DEX (n = 6, median, interquartile range, CXCL10: 2652, 1580–10075 pg/mL; CXCL9: 551.3, 290.6–1153 pg/mL; IFN‐γ: 68.67, 48.40–87.06 pg/mL; neopterin: 256.9, 84.48–413.8 nmol/L) were significantly elevated compared to those in controls (epilepsy patients for cytokines/chemokines, n = 12, NIND patients for neopterin, n = 13), and CXCL10 and neopterin were significantly decreased at post‐IT‐DEX in patients with FIRES (n = 6; CXCL10: 600.5, 230.8–2319 pg/mL; neopterin: 36.35, 23.35–71.88 nmol/L), but CXCL9 and IFN‐γ did not change significantly (CXCL9: 145.3, 102.1–613.8 pg/mL; IFN‐γ: 34.73, 22.81–100.2 pg/mL) (Fig. 3). All four proinflammatory mediators were still higher in patients compared to controls after IT‐DEX.
Figure 3.
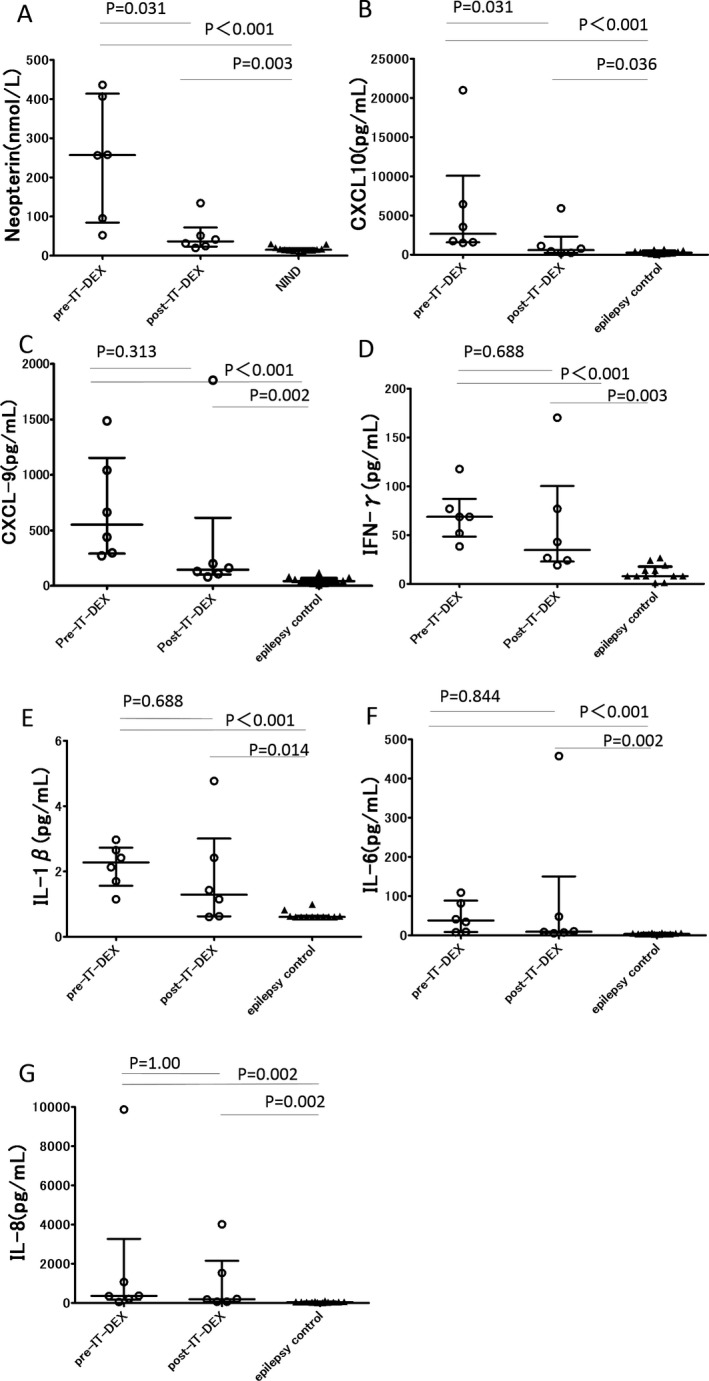
Proinflammatory cytokine/chemokine and neopterin concentrations in CSF from patients with FIRES and controls. (A): CSF neopterin levels were measured in samples from patients with FIRES and patients with noninflammatory neurological disease (NIND) using HPLC. FIRES, N = 6; NIND, N = 13. (B)–(G): CSF levels of (B) CXCL‐10, (C) CXCL‐9, (D) IFN‐γ, (E) IL‐1β, (F) IL‐6, and (G) IL‐8 measured using a multiplexed immunoassay in samples from patients with FIRES and epilepsy. FIRES, N = 6; Epilepsy, N = 12. Each dot represents the cytokine/chemokine or neopterin levels of each patient. Bars express the median and interquartile range.
IL‐1β, IL‐6, and IL‐8 concentrations before IT‐DEX in the FIRES group were significantly elevated compared to controls (n = 12, IL‐1β: median 2.275, interquartile range 1.563–2.73 vs. 0.61, 0.61–0.63 pg/mL, P < 0.001; IL‐6: 37.71, 8.595–88.57 vs. 3.475, 2.930–5.633 pg/mL, P < 0.001; IL‐8: 360.1, 166.0–3268 vs. 44.32, 35.43–57.87 pg/mL, P = 0.002), however, the sequential fluctuation according to IT‐DEX was heterogeneous in the six patients.
Patient 5 and Patient 6 had shown sepsis and renal failure due to interstitial nephritis resulting from continuous hemodialysis, and venous thrombosis, respectively, from before the initiation of IT‐DEX. None of the six patients had a severe adverse event that was determined to be associated with IT‐DEX administration.
Discussion
We administered an intrathecal injection of dexamethasone during the acute or chronic phase of illness in six patients with FIRES. IT‐DEX resulted in early withdrawal of thiopental, an improvement in seizure spreading and background activities on EEG. The median period of mechanical ventilation in the current study was 26.5 days, which was shorter than that for 58 patients included in a previous report, who had a median of 41 days ventilation (mean 49, SD 44.56, range 4–220). 9 Importantly, the shorter the duration between the onset of illness and the introduction of IT‐DEX, the shorter the length of stay in the ICU. Our results showed that IT‐DEX as an add‐on therapy may have a possibility to downgrade intravenous concomitant medications during both the acute and chronic phases and shorten the ICU stay. None of the six patients experienced high‐grade adverse events during IT‐DEX therapy.
To our knowledge, this is the first report on the use of IT‐DEX for FIRES. The effect of systemic administration of steroids on FIRES was often insufficient. In addition, systemic infection or other complications induced by the prolonged use of barbiturates often discourages systemic administration of immuno‐suppressive agents. For example, the first patient in our cohort to whom IT‐DEX was administered showed complication of sepsis and renal failure and was facing a life crisis. Systemic administration of an immunosuppressant had a risk of aggravating those complications. The efficacy and safety of IT‐DEX is warranted by the observation that intrathecal injection of methotrexate and methylprednisolone was effective for anti‐NMDA‐receptor encephalitis. 17 , 18 Another advantage of dexamethasone is its cost‐effectiveness and availability. From this point of view, the tolerability of IT‐DEX in our patients indicated that IT‐DEX is a promising therapy, and further studies are required.
Although pathological studies of FIRES previously revealed little cellular infiltration, recent studies have demonstrated increased proinflammatory cytokine/chemokine levels in the CSF of patients with FIRES. 12 , 19 The therapeutic usage of IT‐DEX for FIRES is justified by the fact that proinflammatory molecules are elevated predominantly in CSF compared to serum in this condition. It is known that IL‐1β is a proinflammatory cytokine that is chiefly involved in both infections and febrile seizures. In addition, IL‐1β, IL‐6, and TNF‐α have been shown to lower the threshold of seizures based on experimental findings in animal models and human studies. 20 , 21 It is hypothesized that inflammation and seizure activity reciprocally induce activation of a vicious cycle of aberrant hyperexcitability. 22 Recently, new therapeutic approaches targeting the immunological mechanisms involved in FIRES are drawing attention since recent reports indicated the potency of the IL‐6 receptor inhibitor tocilizumab, and a recombinant human interleukin‐1 receptor antagonist (IL1RA), anakinra, for FIRES. 23 , 24 Reduced expression of intracellular IL1RA isoforms and a functional deficiency in IL1RA inhibitory activity in patients with FIRES has been shown. 25 Although the pathogenic role of proinflammatory cytokines/chemokines in FIRES remains controversial, the remarkable success of anti‐cytokine treatments provides further support for the disease‐promoting roles of these molecules. However, Anakinra was unavailable and tocilizumab was not approved for the treatment of neurological diseases in Japan.
Our analysis of serial CSF samples revealed that CXCL10 and neopterin approached peak levels within several days to two weeks from the disease onset and then significantly decreased in the majority of our cases after IT‐DEX, but was still above the levels of control patients with epilepsy, including chronic cases. Neopterin is known not only as a pteridin metabolite but also as a biological marker of inflammatory‐immune mediated processes in which T‐helper cell 1 and macrophages are involved, produced by stimulation of interferon‐gamma from activated T cell. In fact, there were a correlation among neopterin, CXCL9, and CXCL10. Neopterin is more stable than IFN‐γ which is degraded within an extremely short time. Its measurement is a simple and useful way for evaluating Th1 system activation. CSF neopterin was reported as a useful early biomarker for immune‐associated CNS diseases (i.e., Influenza‐associated encephalopathy) and has been routinely measured in Osaka City General Hospital since the 1990s. 26 , 27 The elevation of neopterin in CSF was often experienced in FIRES patients.
Second, IL‐1β, IL‐6, and IL‐8 showed a trend toward a decline during IT‐DEX in some patients but did not significantly decrease after IT‐DEX. The production of IL‐1β and IL‐6 is dependent on NF‐κB pathway, whereas CXCL10 and neopterin are associated with JAK‐STAT pathway. Significant reduction of CXCL10 and neopterin in accordance with clinical improvement after IT‐DEX suggests previously unknown involvement of JAK‐STAT pathway in the pathogenesis of FIRES. Glucocorticoid administration in FIRES is reasonable because it suppresses not only NF‐κB but also IFN‐γ signaling pathways. 28
Finally, the mechanisms of action of IT‐DEX may involve cytokine‐independent mechanisms. There is a possibility that dexamethasone contributed to the decreased excitability observed in this study owing not only to its direct actions on cytokine release but also to actions on the downstream cascade and neurodegeneration, by protecting against blood–brain barrier damage, modulating various neurotransmitters, including gamma‐aminobutyric acid (GABA), reducing the production and release of corticotrophin‐releasing hormone in specific brain regions, and reducing cerebral swelling. 29 , 30 , 31 Further studies with larger sample sizes are needed to validate the efficacy of dexamethasone on these proinflammatory molecules. Thirty percent of subjects in this study had a good epileptic prognosis, although functional outcomes measured by the PCPC, which focuses on cognitive impairment, remained unfavorable in all patients. Considering that the cytokine response was prominent at the pretreatment phase, earlier intervention with IT‐DEX may further improve the prognosis of FIRES.
The limitations of our study include the small number of patients, the retrospective, nonrandomized nature of the study, the partly different treatment regimens of IT‐DEX, unstandardized SRSE management, and the lack of an untreated control group. In addition, the delayed effect of the other immunotherapies (IVIG and IVMP) used before IT‐DEX was started or spontaneous remission cannot be excluded. Furthermore, our cases might be biased toward more severe conditions because our facility is a higher medical institution. Lastly, some concomitant treatments were heterogeneous and might influence the efficacy of IT‐DEX. Since the study is retrospective, it was practically unavoidable to change concomitant treatments including antiepileptic agents depending on patients’ condition.
IT‐DEX represents a therapeutic option for patients with FIRES that could shorten the duration of the critical stage of the disease. The effect of IT‐DEX on FIRES might include cytokine‐independent mechanisms. Our proinflammatory data from the CSF provide additional evidence for the involvement of inflammatory mediators in FIRES, however, various factors and processes that link intrathecal overproduction of proinflammatory cytokines/chemokines and electrophysiological excitability in FIRES need to be clarified. Further well‐designed, adequately powered clinical studies are warranted to validate the efficacy of IT‐DEX in FIRES.
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
Author Contributions
AH, IK, HK, SO, MS, and HS contributed to the conception and design of the study. AH, IK, TI, MN, MT, KA, JI, AU, and HS contributed to the acquisition and analysis of the data. AH performed statistical analysis. All authors contributed to drafting the paper and were involved in the approval of the final version.
Funding Information
This work was supported by JSPS KAKENHI, Grant Number 19K08311(HS), and MHLW Research program on rare and intractable diseases, Grant Number JPMH20FC1039
Supporting information
Supplementary Figure S1. Correlation between the duration from the disease onset to the introduction of IT‐DEX and the duration of critical stage in six patients with FIRES. (A): In the scatter plot, the duration from the disease onset to the initiation of IT‐DEX is shown on the X‐axis, and the duration of stay in ICU is shown on the Y‐axis. (B): The duration of mechanical ventilation is plotted against the duration from the disease onset to the initiation of IT‐DEX.
Acknowledgments
We thank Dr. Shizuka Nagase, Dr. Naohiro Yamamoto, Dr. Masataka Fukuoka, Dr. Kiyohiro Kim, Dr. Hitomi Tsuji, Dr. Yuka Hattori, Dr. Yasuyoshi Otsuka, Dr. Hiroshi Rinka, and Dr. Hiroko Matsushita for sample acquisition. We thank Dr. Takako Matsuoka for supporting the cytokine assay.
Funding Statement
This work was funded by JSPS KAKENHI grant 19K08311; MHLW Research program on rare and intractable diseases grant JPMH20FC1039.
Contributor Information
Ichiro Kuki, Email: i-kuki@med.osakacity-hp.or.jp.
Hiroshi Sakuma, Email: sakuma-hs@igakuken.or.jp.
References
- 1. van Baalen A, Hausler M, Boor R, et al. Febrile infection‐related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia 2010;51:1323–1328. [DOI] [PubMed] [Google Scholar]
- 2. Sakuma H, Awaya Y, Shiomi M, et al. Acute encephalitis with refractory, repetitive partial seizures (AERRPS): a peculiar form of childhood encephalitis. Acta Neurol Scand 2010;121:251–256. [DOI] [PubMed] [Google Scholar]
- 3. Wilder‐Smith EP, Lim EC, Teoh HL, et al. The NORSE (new‐onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singapore 2005;34:417–420. [PubMed] [Google Scholar]
- 4. Mikaeloff Y, Jambaque I, Hertz‐Pannier L, et al. Devastating epileptic encephalopathy in school‐aged children (DESC): a pseudo encephalitis. Epilepsy Res 2006;69:67–79. [DOI] [PubMed] [Google Scholar]
- 5. Gaspard N, Foreman BP, Alvarez V, et al. New‐onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology 2015;85:1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaspard N, Hirsch LJ, Sculier C, et al. New‐onset refractory status epilepticus (NORSE) and febrile infection‐related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia 2018;59:745–752. [DOI] [PubMed] [Google Scholar]
- 7. Nabbout R, Mazzuca M, Hubert P, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES). Epilepsia 2010;51:2033–2037. [DOI] [PubMed] [Google Scholar]
- 8. van Baalen A, Vezzani A, Hausler M, Kluger G. Febrile infection‐related epilepsy syndrome: clinical review and hypotheses of epileptogenesis. Neuropediatrics 2017;48:5–18. [DOI] [PubMed] [Google Scholar]
- 9. Kramer U, Chi CS, Lin KL, et al. Febrile infection‐related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia 2011;52:1956–1965. [DOI] [PubMed] [Google Scholar]
- 10. Lee HF, Chi CS. Febrile infection‐related epilepsy syndrome (FIRES): therapeutic complications, long‐term neurological and neuroimaging follow‐up. Seizure 2018;56:53–59. [DOI] [PubMed] [Google Scholar]
- 11. Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new‐onset refractory status epilepticus (NORSE), febrile infection‐related epilepsy syndrome (FIRES), and related conditions. Epilepsia 2018;59:739–744. [DOI] [PubMed] [Google Scholar]
- 12. Sakuma H, Tanuma N, Kuki I, et al. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection‐related refractory status epilepticus. J Neurol Neurosurg Psychiatry 2015;86:820–822. [DOI] [PubMed] [Google Scholar]
- 13. Mizuguchi M, Ichiyama T, Imataka G, et al. Guidelines for the diagnosis and treatment of acute encephalopathy in childhood. Brain Dev 2020; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Shiomi M, Togawa M, Kurimasa H, et al. Neopterin concentrations of serum and cerebrospinal fluid in febrile convulsion, aseptic meningitis and influenza encephalopathy. Pteridines 1999;10:31–37. [Google Scholar]
- 15. Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 16. Mohammad SS, Soe SM, Pillai SC, et al. Etiological associations and outcome predictors of acute electroencephalography in childhood encephalitis. Clin Neurophysiol 2016;127:3217–3224. [DOI] [PubMed] [Google Scholar]
- 17. Tatencloux S, Chretien P, Rogemond V, et al. Intrathecal treatment of anti‐N‐Methyl‐D‐aspartate receptor encephalitis in children. Dev Med Child Neurol 2015;57:95–99. [DOI] [PubMed] [Google Scholar]
- 18. Yang XZ, Zhu HD, Ren HT, et al. Utility and safety of intrathecal Methotrexate treatment in severe anti‐N‐methyl‐ D‐aspartate receptor encephalitis. A pilot study. Chin Med J 2018;131:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kothur K, Bandodkar S, Wienholt L, et al. Etiology is the key determinant of neuroinflammation in epilepsy: elevation of cerebrospinal fluid cytokines and chemokines in febrile infection‐related epilepsy syndrome and febrile status epilepticus. Epilepsia 2019;60:1678–1688. [DOI] [PubMed] [Google Scholar]
- 20. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol 2011;7:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol 2012;33:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nabbout R, Vezzani A, Dulac O, Chiron C. Acute encephalopathy with inflammation‐mediated status epilepticus. Lancet Neurol 2011;10:99–108. [DOI] [PubMed] [Google Scholar]
- 23. Jun JS, Lee ST, Kim R, et al. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol 2018;84:940–945. [DOI] [PubMed] [Google Scholar]
- 24. Kenney‐Jung DL, Vezzani A, Kahoud RJ, et al. Febrile infection‐related epilepsy syndrome treated with anakinra. Ann Neurol 2016;80:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clarkson BDS, LaFrance‐Corey RG, Kahoud RJ, et al. Functional deficiency in endogenous interleukin‐1 receptor antagonist in patients with febrile infection‐related epilepsy syndrome. Ann Neurol 2019;85:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dale RC, Brilot F, Fagan E, Earl J. Cerebrospinal fluid neopterin in paediatric neurology: a marker of active central nervous system inflammation. Dev Med Child Neurol 2009;51:317–323. [DOI] [PubMed] [Google Scholar]
- 27. Macdonald‐Laurs E, Koirala A, Britton PN, et al. CSF neopterin, a useful biomarker in children presenting with influenza associated encephalopathy? Eur J Paediatr Neurol 2019;23:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu X, Li WP, Meng C, Ivashkiv LB. Inhibition of IFN‐gamma Signaling by Glucocorticoids. J Immunol. 2003;170:4833–4839. [DOI] [PubMed] [Google Scholar]
- 29. Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 2005;46:1724–1743. [DOI] [PubMed] [Google Scholar]
- 30. Marchi N, Granata T, Ghosh C, Janigro D. Blood‐brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia 2012;53:1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Araki T, Otsubo H, Makino Y, et al. Efficacy of dexamethasone on cerebral swelling and seizures during subdural grid EEG recording in children. Epilepsia 2006;47:176–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Correlation between the duration from the disease onset to the introduction of IT‐DEX and the duration of critical stage in six patients with FIRES. (A): In the scatter plot, the duration from the disease onset to the initiation of IT‐DEX is shown on the X‐axis, and the duration of stay in ICU is shown on the Y‐axis. (B): The duration of mechanical ventilation is plotted against the duration from the disease onset to the initiation of IT‐DEX.


