Abstract
Several studies have suggested that mediastinitis is a strong predictor for poor long‐term survival after coronary artery bypass surgery (CABG). In those studies, several conventional wound‐healing techniques were used. Previously, we have shown no difference in long‐term survival between CABG patients with topical negative pressure (TNP)‐treated mediastinitis and CABG patients without mediastinitis. The present study was designed to elucidate if TNP, applied over the myocardium, resulted in an increase of the total amount of coronary blood flow. Six pigs underwent median sternotomy. The coronary blood flow was measured, before and after the application of TNP (−50 mmHg), using coronary electromagnetic flow meter probes. Analyses were performed before left anterior descending artery (LAD) occlusion (normal myocardium) and after 20 minutes of LAD occlusion (ischaemic myocardium). Normal myocardium: 171·3 ± 14·5 ml/minute before to 206·3 ± 17·6 ml/minute after TNP application, P < 0·05. Ischaemic myocardium: 133·7 ± 18·4 ml/minute before to 183·2 ± 18·9 ml/minute after TNP application, P < 0·05. TNP of −50 mmHg applied over the LAD region induced a significant increase in the total coronary blood flow in both normal and ischaemic myocardium.
Keywords: Coronary blood flow, Poststernotomy mediastinitis, Topical negative pressure (TNP)
Introduction
Topical negative pressure (TNP) has been shown to facilitate the healing of chronic and problematic wounds 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. The physiological and molecular biological mechanisms by which TNP promotes wound healing are still largely unknown. However, TNP is known to stimulate blood flow in tissues such as subcutaneous tissue and skeletal muscle 11, 12, 13. TNP produces mechanical stress and a pressure gradient across the tissue, which cause a surge of blood to the area. Mechanical forces and increased blood flow are known to stimulate granulation tissue formation, that is endothelial proliferation, capillary budding and angiogenesis 14, 15, 16.
Several studies have suggested that mediastinitis is a strong predictor for poor long‐term survival after coronary artery bypass surgery (CABG) 17, 18, 19, 20, 21, 22. Braxton and coworkers showed in a large study including 36 078 patients after isolated CABG that actuarial survival after 10 years was 39% in patients with mediastinitis and 70% in patients without mediastinitis (20). Milano et al. have suggested that mediastinitis may cause negative long‐terms effects on several organs such as the heart and kidneys (19). Theoretically, a massive immunological response during a prolonged period of infection may cause adverse effects on bypass grafts. In those studies, reporting poor long‐term survival after mediastinitis, several conventional wound‐healing techniques were used (closed irrigation, delayed wound closure or reconstructing with omentum or pectoral flaps).
Previously, we have showed no difference in long‐term survival between CABG patients with TNP‐treated mediastinitis and CABG patients without mediastinitis (23). This indicates that these patients might have developed increased coronary collateral blood vessels during TNP and may therefore be better prepared when bypass grafts fail to work. It may be that the TNP stimulation of blood flow and development of collateral blood vessels in part account for the reduced long‐term mortality in patients treated with TNP for poststernotomy mediastinitis after CABG.
We have earlier shown that TNP of −50 mmHg significantly increases microvascular blood flow in the underlying myocardium (24). The present study was designed to elucidate if the increase in microvascular blood flow is a result of an increase of the total coronary blood flow or a redistribution of blood from other areas of the heart. An unchanged or decreased amount of the total coronary blood flow to the heart might theoretically cause ischaemia in parts of the myocardium not exposed to TNP. However, an increase in total coronary blood flow will stimulate granulation tissue formation, that is endothelial proliferation, capillary budding and angiogenesis. No such study has to our knowledge been performed before.
Materials and methods
Experimental animals
A porcine model was use for the present study. Six domestic landrace pigs of both genders, with a mean body weight of 70 kg, were fasted overnight with free access to water. The study was approved by the Ethics Committee for Animal Research, Lund University, Sweden. The investigation complied with the ‘Guide for the Care and Use of Laboratory Animals’ as recommended by the U.S. National Institutes of Health and published by the National Academies Press (1996).
Anaesthesia
All animals were premedicated intramuscularly with ketamine (30 mg/kg) before they were brought into the laboratory. Before commencing surgery, sodium thiopental (5 mg/kg), atropine (0·02 mg/kg) and pancuronium (0·5 mg/kg) were given intravenously. Tracheotomy was performed with a Portex endotracheal tube (7·5‐mm internal diameter, Medcompare™, San Francisco, CA, USA). A servo‐ventilator (Siemens Elema 300A, Stockholm, Sweden) was used for mechanical ventilation throughout the experiment. The ventilator settings used were minute volume = 100 ml/kg, FiO2 = 0·5, breathing frequency = 16 breaths/minute and positive end‐expiratory pressure = 5 cm H2O.
Anaesthesia and muscular paralysis were maintained with a continuous intravenous infusion of 8–10 mg/kg/hour propofol (Diprivan®; AstraZeneca, Sodertalje, Sweden), 0·15 mg/kg/hour fentanyl (Leptanal®; Lilly, Solna, Sweden) and 0·6 mg/kg/hour pancuronium (Pavulon®; Organon Teknika, Boxtel, the Netherlands).
Data acquisition
Mean arterial pressure, central venous pressure, heart frequency and ventilatory parameters were recorded throughout the experiments.
Surgical procedure
Surgery was performed through median sternotomy. After heparinisation (400 IU/kg), a cardiopulmonary bypass (CPB) was installed with an arterial cannula [22 French, DLP® Elongated One‐Piece Arterial Cannula (EOPA™); Medtronic Inc., Minneapolis, MO] in the distal ascending aorta and a venous cannula (32 French, MC2® Two‐Stage Venous Cannula, also from Medtronic Inc.) inserted through the right atrium. Before cannulation of the heart, the cannulae were inserted through the thoracic wall to prevent air leakage during TNP application. CPB was conducted in normothermia. Ventricular fibrillation was subsequently induced in the heart. No aortic cross‐clamping was performed and no cardioplegia was used. The mean arterial pressure was maintained between 60 and 80 mmHg. A left ventricular vent (DLP® Vent, also from Medtronic Inc.) was used to protect the left chamber from overloading. Pulmonary ventilation was applied at a rate of 4 l/minute during the experiments. Coronary pulmonary bypass prevents the risk of circulatory failure during left anterior descending artery (LAD) occlusion, thereby facilitating experimental analysis in the case of the ischaemic myocardium.
Coronary electromagnetic flow meter probes
The coronary electromagnetic flow meter probes (model BL 613; Biotronex Laboratory Inc., Chester, MD) were positioned around the proximal part of the three coronary vessels: LAD, circumflex coronary artery (CCX) and right coronary artery (RCA), respectively. The coronary blood flow was measured continuously throughout the experiments. Calibration of each probe was checked in vitro at the end of each experiment.
A round hole with a diameter of 5 cm was made in the middle of a phrenic nerve pad (Phrenic Nerve Pad®; Medtronic Inc.) and placed on top of the heart. The pad was stabilised to the surrounding myocardium with eight to ten sutures (Prolene 5‐0; Ethicon Inc., Somerville, NJ, USA) and to the posterior sternal edges with sutures (Dermalon 2‐0; Davis and Geck, St Louis, MO). A retractor was used throughout the experiments to keep the sternal edges apart. A polyurethane foam dressing, with an open pore structure of 400–600 μm (KCI, Copenhagen, Denmark), was placed between the sternal edges. The foam was continuously sutured to the surrounding skin (Dermalon 2‐0; Davis and Geck). The wound was sealed with a transparent adhesive drape. A track pad (KCI) was inserted through the drape and was connected to a vacuum pump, (V.A.C. pump unit, KCI). When the negative pressure is applied, the heart will be drawn up towards the phrenic nerve pad and the foam without interfering with the sternal edges. This procedure causes the application of negative pressure to affect only the myocardium exposed by the 5‐cm‐diameter hole.
Experimental protocol
The coronary blood flow was measured continuously by electromagnetic flow meter probes. Recordings were made in normal myocardium before and while a negative pressure of −50 mmHg was applied.
The LAD was then occluded for 20 minutes with an elastic vessel loop. Coronary blood flow was then measured before and after 20 minutes of occlusion. A negative pressure of −50 mmHg was then applied to the myocardium, and coronary blood flow changes were recorded. The negative pressure was then removed.
Statistics
Calculations and statistical analysis were performed using GraphPad 4.0 software. Statistical analysis was performed using Student’s paired t‐test. Significance was defined as P < 0·05 and non significant (n.s.) at P > 0·05. Values are presented as means ± standard error of the mean.
Results
Normal myocardium
A TNP of −50 mmHg induced an immediate, significant increase in total coronary blood flow in normal myocardium (171·3 ± 14·5 ml/minute before to 206·3 ± 17·6 ml/minute after TNP application, P < 0·05) (Figure 1). Divided between the three coronary arteries, −50 mmHg induced an immediate increase in local coronary blood flow in the CCX (49·2 ± 6·1 ml/minute before to 53·7 ± 5·0 ml/minute after TNP application, n.s. P > 0·05), the LAD (60·5 ± 11·1 ml/minute before to 77·8 ± 12·2 ml/minute after TNP application, P < 0·05) and the RCA (61·7 ± 11·6 ml/minute before to 74·8 ± 13·1 ml/minute after TNP application, P < 0·05) (Figure 2).
Figure 1.
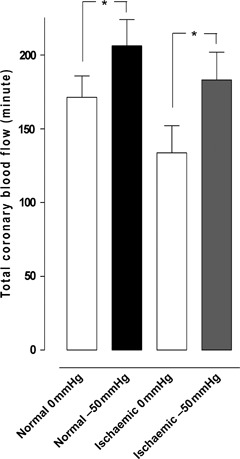
The total blood flow changes using coronary electromagnetic flow meter probes in normal and ischaemic myocardium before and during application of −50 mmHg. The measurements were performed at the proximal part of the right coronary artery, the left anterior descending artery and the circumflex coronary artery in six pigs. The change in total coronary artery blood flow is shown as mean values ± standard error of the mean. Statistical analysis was performed using Student’s paired t‐test, and significance was defined as *P < 0·05 and not significant (n.s.) at P > 0·05.
Figure 2.
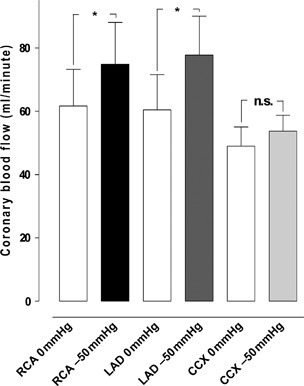
The blood flow measured using coronary electromagnetic flow meter probes in normal myocardium. The measurements were performed at the proximal part of the right coronary artery (RCA), left anterior descending artery (LAD) and circumflex coronary artery (CCX) in the myocardium in six pigs, with a topical negative pressure of −50 mmHg. The results are shown as mean values ± standard error of the mean. Statistical analysis was performed using Student’s paired t‐test, and significance was defined as *P < 0·05 and not significant (n.s.) at P > 0·05. occl, occlusion.
LAD occlusion
Ischaemia was induced by occlusion of the LAD for 20 minutes. The coronary blood flow in the CCX increased (from 47·8 ± 8·4 ml/minute before to 56·3 ± 5·6 ml/minute after TNP application, n.s. P > 0·05). The coronary blood flow in the LAD decreased significantly (from 74·3 ± 9·8 ml/minute before to 13·3 ± 2·0 ml/minute, P < 0·05) after 20 minutes of LAD occlusion. The coronary blood flow in the RCA increased (from 65·8 ± 14·6 ml/minute before to 67·5 ± 14·7 ml/minute after TNP application, n.s. P > 0·05; Figure 3).
Figure 3.
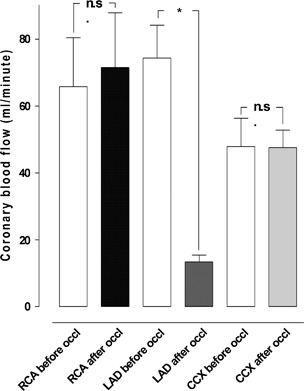
The blood flow measured using coronary electromagnetic flow meter probes before and after 20 minutes of left anterior descending artery (LAD) occlusion. The measurements were performed at the proximal part of the right coronary artery (RCA), LAD and circumflex coronary artery (CCX) in six pigs. Results are shown as mean values ± standard error of the mean. Statistical analysis was performed using Student’s paired t‐test, and significance was defined as *P < 0·05 and not significant (n.s.) at P > 0·05.
Ischaemic myocardium
A TNP of −50 mmHg induced an immediate, significant increase in total coronary artery blood flow in ischaemic myocardium (133·7 ± 18·4 ml/minute before to 183·2 ± 18·9 ml/minute after TNP application, P < 0·05; Figure 1). Application of −50 mmHg induced an immediate increase in local coronary blood flow in the CCX (52·2 ± 4·2 ml/minute before to 69·2 ± 7·0 ml/minute after TNP application, n.s. P > 0·05). The coronary blood flow in the LAD significantly increased (from 15·0 ± 2·4 ml/minute before to 21·0 ± 7·9 ml/minute after TNP application, P < 0·05), and the coronary blood flow in RCA also significantly increased (from 66·2 ± 17·9 ml/minute before to 93·0 ± 15·7 ml/minute after TNP application, P < 0·05; Figure 4).
Figure 4.
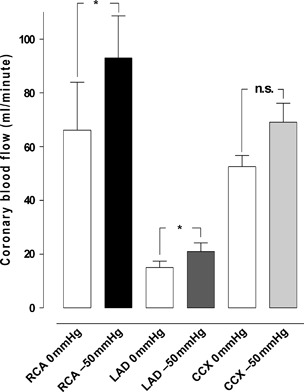
The blood flow measured using coronary electromagnetic flow meter probes in ischaemic myocardium. The measurements were performed at the proximal part of the right coronary artery (RCA), the left anterior descending artery (LAD) and the circumflex coronary artery (CCX) in the myocardium in six pigs, with a topical negative pressure of −50 mmHg. The results are shown as mean values ± standard error of the mean. Statistical analysis was performed using Student’s paired t‐test, and significance was defined as *P < 0·05 and not significant (n.s.) at P > 0·05.
Discussion
Poststernotomy mediastinitis is a rare but potentially lethal complication following cardiac surgery 17, 19, 25. Late TNP has become the therapy of choice for mediastinitis because of the exceptional clinical outcome 6, 7, 10, 23. TNP acts by a subatmospheric pressure application over the wound by controlled suction through a porous dressing. However, the fundamental scientific mechanism of TNP is only partially understood. One of the known effects of TNP is enhanced blood flow to the wound edge and granulation tissue formation 1, 2, 15. TNP increases blood flow velocity and opens up the capillary beds. Mechanical forces exerted by TNP and increased blood flow affect the cytoskeleton in the vascular cells, stimulating endothelial proliferation, capillary budding and angiogenesis, that is granulation tissue formation 14, 16, 26, 27.
In patients with poststernotomy mediastinitis treated with TNP, the TNP is in direct contact with the heart, which is exposed through the diastase of the sternotomy. We have previously observed that these patients develop a richly vascularised granulation tissue over the heart within 6–7 days on the exposed surface of the heart. In the present study, we hypothesised that the application of TNP to the surface of the heart would stimulate neovascularisation and microvascular blood flow in the myocardium, as seen in skeletal muscle during TNP 13, 28, 29, 30, 31. It is commonly known among cardiothoracic surgeons that mediastinitis is a strong predictor of poor long‐term survival after CABG, using conventional wound‐healing techniques (such as closed irrigation, delayed wound closure or reconstructing with omentum or pectoral flaps). It has been suggested that mediastinitis may cause negative long‐term effects on several organs, such as the heart and kidneys 18, 20, 32. Previously, we have showed no difference in long‐term survival between CABG patients with TNP‐treated mediastinitis and CABG patients without mediastinitis (23). This indicates that these patients might have developed increased coronary collateral blood vessels during TNP and may therefore be better prepared when bypass grafts fail to work. It may be that the TNP stimulation of blood flow and development of collateral blood vessels in part account for the reduced long‐term mortality in patients treated with TNP for poststernotomy mediastinitis after CABG.
We have earlier shown that TNP of −50 mmHg significantly increases microvascular blood flow in the underlying myocardial tissue (24). In the present study, we show that application of a TNP of −50 mmHg directly on the myocardium (over the LAD region) results in an increase of the total coronary blood flow not only in normal but also in ischaemic myocardium (Figure 4). An unchanged or decreased amount of the total coronary blood flow might theoretically cause ischaemia in parts of the myocardium not exposed to TNP. Because the present study shows a significant increase in the total amount of coronary blood flow, ischaemic areas are not likely to occur.
Therapeutic angiogenesis, wherein exogenous growth factors are administered to ischaemic tissue to enhance the reperfusion of these tissues, has been investigated as a potential therapy for patients with advanced coronary artery disease as an alternative to conventional treatment such as percutaneous coronary intervention (PCI) and CABG. It includes both protein and gene therapy, and they have both successfully induced angiogenic responses in animal studies, but optimal delivery of the angiogenic factor is difficult 33, 34. However, vascular endothelia growth factor (VEGF) proteins have been shown to play a key role in the modulation of angiogenesis and vascular growth 35, 36. Moreover, TNP produces a mechanical shear stress that is known to activate VEGF 37, 38, 39, 40, 41.
In the present study, we show that topically applied negative pressure of −50 mmHg over the LAD region results in a significant increase of the total amount of coronary blood flow to the heart muscle. This might in part explain the improved long‐term outcome using TNP treatment on patients with poststernotomy mediastinitis compared with conventional therapy presented in an earlier report (23).
Acknowledgements
We would like to thank Johan Ingemansson (Statistical Solutions IP) for his expert contribution to the statistic analyses. This study was supported by Anders Otto Swärd’s Foundation/Ulrika Eklund’s Foundation, Anna Lisa and Sven Eric Lundgren’s Foundation for medical research, the Åke Wiberg Foundation, the M. Bergvall Foundation, the Swedish Medical Association, the Royal Physiographic Society in Lund, the Swedish Medical Research Council, the Crafoord Foundation, the Swedish Heart‐Lung Foundation, the Swedish Government Grant for Clinical Research and the Swedish Hypertension Society.
References
- 1. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 2. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76; discussion 77. [PubMed] [Google Scholar]
- 3. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 4. O’Connor J, Kells A, Henry S, Scalea T. Vacuum‐assisted closure for the treatment of complex chest wounds. Ann Thorac Surg 2005;79:1196–200. [DOI] [PubMed] [Google Scholar]
- 5. Brown KM, Harper FV, Aston WJ, O’Keefe PA, Cameron CR. Vacuum‐assisted closure in the treatment of a 9‐year‐old child with severe and multiple dog bite injuries of the thorax. Ann Thorac Surg 2001;72:1409–10. [DOI] [PubMed] [Google Scholar]
- 6. Gustafsson RI, Sjogren J, Ingemansson R. Deep sternal wound infection: a sternal‐sparing technique with vacuum‐assisted closure therapy. Ann Thorac Surg 2003;76:2048–53; discussion 53. [DOI] [PubMed] [Google Scholar]
- 7. Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum‐assisted closure versus conventional treatment. Ann Thorac Surg 2005;79:2049–55. [DOI] [PubMed] [Google Scholar]
- 8. Gustafsson R, Johnsson P, Algotsson L, Blomquist S, Ingemansson R. Vacuum‐assisted closure therapy guided by C‐reactive protein level in patients with deep sternal wound infection. J Thorac Cardiovasc Surg 2002;123:895–900. [DOI] [PubMed] [Google Scholar]
- 9. Cowan KN, Teague L, Sue SC, Mahoney JL. Vacuum‐assisted wound closure of deep sternal infections in high‐risk patients after cardiac surgery. Ann Thorac Surg 2005;80:2205–12. [DOI] [PubMed] [Google Scholar]
- 10. Fleck TM, Fleck M, Moidl R, Czerny M, Koller R, Giovanoli P, Hiesmayer MJ, Zimpfer D, Wolner E, Grabenwoger M. The vacuum‐assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thorac Surg 2002;74:1596–600; discussion 600. [DOI] [PubMed] [Google Scholar]
- 11. Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 2004;12:600–6. [DOI] [PubMed] [Google Scholar]
- 12. Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum‐assisted closure on wound microcirculation: an experimental study. Asian J Surg 2005;28:211–7. [DOI] [PubMed] [Google Scholar]
- 13. Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum‐assisted closure therapy. Ann Thorac Surg 2005;79:1724–30; discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 14. Vandenburgh HH. Mechanical forces and their second messengers in stimulating cell growth in vitro. Am J Physiol 1992;262:R350–5. [DOI] [PubMed] [Google Scholar]
- 15. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086–96; discussion 97–8. [DOI] [PubMed] [Google Scholar]
- 16. Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, Orgill DP. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006;56:418–22. [DOI] [PubMed] [Google Scholar]
- 17. Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid‐term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 2003;23:943–9. [DOI] [PubMed] [Google Scholar]
- 18. Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, Stewart RW, Golding LA, Taylor PC. J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49:179–86; discussion 86–7. [DOI] [PubMed] [Google Scholar]
- 19. Milano CA, Kesler K, Archibald N, Sexton DJ, Jones RH. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long‐term survival. Circulation 1995;92:2245–51. [DOI] [PubMed] [Google Scholar]
- 20. Braxton JH, Marrin CA, McGrath PD, Ross CS, Morton JR, Norotsky M, Charlesworth DC, Lahey SJ, Clough RA, O’Connor GT; Northern New England Cardiovascular Disease Study Group. Mediastinitis and long‐term survival after coronary artery bypass graft surgery. Ann Thorac Surg 2000;70:2004–7. [DOI] [PubMed] [Google Scholar]
- 21. Toumpoulis IK, Anagnostopoulos CE, Derose JJ Jr, Swistel DG. The impact of deep sternal wound infection on long‐term survival after coronary artery bypass grafting. Chest 2005;127:464–71. [DOI] [PubMed] [Google Scholar]
- 22. Stahle E, Tammelin A, Bergstrom R, Hambreus A, Nystrom SO, Hansson HE. Sternal wound complications – incidence, microbiology and risk factors. Eur J Cardiothorac Surg 1997;11:1146–53. [DOI] [PubMed] [Google Scholar]
- 23. Sjogren J, Nilsson J, Gustafsson R, Malmsjo M, Ingemansson R. The impact of vacuum‐assisted closure on long‐term survival after post‐sternotomy mediastinitis. Ann Thorac Surg 2005;80:1270–5. [DOI] [PubMed] [Google Scholar]
- 24. Lindstedt S, Malmsjo M, Ingemansson R. Blood flow changes in normal and ischemic myocardium during topically applied negative pressure. Ann Thorac Surg 2007;84:568–73. [DOI] [PubMed] [Google Scholar]
- 25. Braxton JH, Marrin CA, McGrath PD, Morton JR, Norotsky M, Charlesworth DC, Lahey SJ, Clough R, Ross CS, Olmstead EM, O’Connor GT. 10‐year follow‐up of patients with and without mediastinitis. Semin Thorac Cardiovasc Surg 2004;16:70–6. [DOI] [PubMed] [Google Scholar]
- 26. Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci 1999;112(Pt 19):3249–58. [DOI] [PubMed] [Google Scholar]
- 27. Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog 1998;14:356–63. [DOI] [PubMed] [Google Scholar]
- 28. Petzina R, Gustafsson L, Mokhtari A, Ingemansson R, Malmsjo M. Effect of vacuum‐assisted closure on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur J Cardiothorac Surg 2006;30:85–9. [DOI] [PubMed] [Google Scholar]
- 29. Chen SZ, Cao DY, Li JQ, Tang SY. [Effect of vacuum‐assisted closure on the expression of proto‐oncogenes and its significance during wound healing]. Zhonghua Zheng Xing Wai Ke Za Zhi 2005;21:197–200. [PubMed] [Google Scholar]
- 30. Wackenfors A, Sjogren J, Algotsson L, Gustafsson R, Ingemansson R, Malmsjo M. The effect of vacuum‐assisted closure therapy on the pig femoral artery vasomotor responses. Wound Repair Regen 2004;12:244–51. [DOI] [PubMed] [Google Scholar]
- 31. Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol 1991;260:H862–8. [DOI] [PubMed] [Google Scholar]
- 32. De Feo M, Renzulli A, Ismeno G, Gregorio R, Della Corte A, Utili R, Cotrufo M. Variables predicting adverse outcome in patients with deep sternal wound infection. Ann Thorac Surg 2001;71:324–31. [DOI] [PubMed] [Google Scholar]
- 33. Yau TM, Kim C, Li G, Zhang Y, Fazel S, Spiegelstein D, Weisel RD, Li RK. Enhanced angiogenesis with multimodal cell‐based gene therapy. Ann Thorac Surg 2007;83:1110–9. [DOI] [PubMed] [Google Scholar]
- 34. Kim C, Li RK, Li G, Zhang Y, Weisel RD, Yau TM. Effects of cell‐based angiogenic gene therapy at 6 months: persistent angiogenesis and absence of oncogenicity. Ann Thorac Surg 2007;83:640–6. [DOI] [PubMed] [Google Scholar]
- 35. Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR, Isom OW, Crystal RG. Six‐month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann Surg 1999;230:466–70; discussion 70–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, Hachamovitch R, Szulc M, Kligfield PD, Okin PM, Hahn RT, Devereux RB, Post MR, Hackett NR, Foster T, Grasso TM, Lesser ML, Isom OW, Crystal RG. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation 1999;100:468–74. [DOI] [PubMed] [Google Scholar]
- 37. Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem 1999;274:18393–400. [DOI] [PubMed] [Google Scholar]
- 38. Detmar M, Brown LF, Berse B, Jackman RW, Elicker BM, Dvorak HF, Claffey KP. Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J Invest Dermatol 1997;108:263–8. [DOI] [PubMed] [Google Scholar]
- 39. Shyu KG, Chang ML, Wang BW, Kuan P, Chang H. Cyclical mechanical stretching increases the expression of vascular endothelial growth factor in rat vascular smooth muscle cells. J Formos Med Assoc 2001;100:741–7. [PubMed] [Google Scholar]
- 40. Milkiewicz M, Mohammadzadeh F, Ispanovic E, Gee E, Haas TL. Static strain stimulates expression of matrix metalloproteinase‐2 and VEGF in microvascular endothelium via JNK‐ and ERK‐dependent pathways. J Cell Biochem 2007;100:750–61. [DOI] [PubMed] [Google Scholar]
- 41. Seko Y, Seko Y, Takahashi N, Shibuya M, Yazaki Y. Pulsatile stretch stimulates vascular endothelial growth factor (VEGF) secretion by cultured rat cardiac myocytes. Biochem Biophys Res Commun 1999;254:462–5. [DOI] [PubMed] [Google Scholar]


