Abstract
Introduction:
It is unclear whether quantifying muscle endurance adds nonredundant information useful for the care of patients with muscular disease.
Methods:
Records were retrospectively reviewed for all Johns Hopkins Myositis Center patients with a muscle endurance assessment (n=128, 226 patient-visits). Muscle endurance and strength were quantified with the Myositis Functional Index-2 (FI2) and manual muscle testing (MMT), respectively.
Results:
Composite FI2 muscle endurance scores were comparable in inclusion body myositis (n=58), dermatomyositis (n=31), and polymyositis (n=39). Overall, muscle endurance correlated with and evolved similarly to strength, inversely to serum creatine kinase. However, in patients with normal or near-normal strength (mean MMT >9.75/10), muscle endurance was typically abnormal and highly variable (mean FI2, 5.6/10; interquartile range, 3.3–7.8/10).
Discussion:
Muscle endurance testing may identify muscle impairment inadequately described by MMT, particularly in patients with high MMT scores.
Keywords: myositis, neuromuscular diseases, muscle strength, physical endurance, functional index
INTRODUCTION
The inflammatory myopathies are a heterogeneous group of autoimmune muscle diseases characterized by autoantibodies, muscle inflammation, and muscle weakness 1. Manual muscle testing (MMT), a measure of muscle strength which assesses capacity of a joint to generate maximal torque against an isometric resistance, is commonly used in myositis patients as a surrogate measure for muscle function.. However, MMT may be limited by a ceiling effect (i.e. lack sensitivity to change) in patients with high baseline strength or with mild muscle weakness 2,3. In addition, in our collective clinical experience, patients with myositis often complain of easy fatigability and/or poor endurance, characteristics which are not evaluated by MMT. Thus, alternative objective tests of muscle function in these patients may augment efforts to monitor disease progression and/or response to therapy.
The myositis functional index-2 (FI2) was developed to quantify endurance in multiple muscle groups and has been validated as correlating with disease activity in dermatomyositis (DM) and polymyositis (PM) patients 2,3. Notably, the FI2 does not have the ceiling effect observed with MMT, and recent reports indicate that FI2 is highly associated with functional status 4,3 and changes over time in response to disease modifying therapies 5,3. Thus, the FI2 may have a role in monitoring muscle function in myositis patients. However, it is unclear if FI2 identifies impairments in muscle function that MMT does not, if FI2 has validity in sporadic inclusion body myositis (IBM), or if FI2, MMT, and serum creatine kinase (CK) levels evolve similarly over time. To explore these questions, we reviewed the records of a single-center cohort of myositis patients who had at least one FI2 evaluation.
METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
This study was conducted under Institutional Review Board-approved protocols with the Johns Hopkins University School of Medicine. Written informed consent for research from all patients was obtained.
Patients and Review of Medical Records
All patients from the Johns Hopkins Myositis Center Longitudinal Cohort were included in this study if they had at least one FI2 evaluation. To avoid a biased dataset including more severely ill patients, broad myositis diagnostic criteria were utilized. Patients were considered to have DM or PM based on Bohan and Peter criteria 6 and IBM patients met the 2011 European Neuromuscular Centre criteria for probable or definite IBM 7.
Muscle Function Quantification
Standard MMT strength testing was performed by each patient’s attending physician, with data transformed to a 1–10 scale using the Kendall conversion, as previously described (i.e. 5/5 strength would transform to 10/10, 4+ to 9/10, and so forth) 8,9. The FI2 was tested as described 2; briefly, patients were instructed to complete as many repetitions as possible (up to a defined maximum) at a predetermined number of repetitions per minute. Repetitions were counted for seven different standardized movements—neck extension (“head lift”), plantarflexion (“heel lift”), dorsiflexion (“toe lift”), shoulder flexion, shoulder abduction, hip flexion, and stepping to a 25cm high stool (“step test”)—the latter four of which were performed bilaterally. Repetitions were not counted if full range of motion was not attained. To limit variability, FI2 tests were done exclusively by two physical therapists trained to follow the same protocol. Although not systematically recorded, rating of perceived exertion scores were reported to the clinician after each movement to assess motivation to complete FI2 testing. Patients were excluded from analysis in the rare case of FI2 failure despite low exertion. To better facilitate score comparisons with MMT (0–10 scale), FI2 scores for each exercise were calculated as a percentage of maximum repetitions possible and divided by 10; for example, 30 repetitions performed out of a maximum 60 garnered a score of 5/10. Composite FI2 and MMT scores were defined as the mean of all individual FI2 and MMT variables, respectively, with bilateral test components (e.g. left hip flexion, right hip flexion) first averaged to form one variable. Individual FI2 movement scores were used for floor and ceiling effect analyses (described below) and composite FI2 and MMT scores were used for all other comparisons.
Statistics and Data Visualization
FI2 components were determined to have a ceiling or floor effect if the median group score was the maximum or minimum possible score, respectively. Scatter and boxplots were produced using Seaborn version 0.7.1. To visualize evolution of study variables over time, locally-weighted regression was performed (LOWESS), with the corresponding graphs produced in STATA MP 13.1. To determine significance of the relationship between CK, FI2, and MMT accounting for the different number of observations per patient, we used a mixed effect multilevel model with random intercepts. Spearman’s rank correlation was used to determine associations between FI2 subscores and composite FI2 scores. The two-sided t-test was used to compare single variables between study groups. P values of less than 0.05 were considered significant.
RESULTS
Distribution of FI2 scores in DM, PM, and IBM patients
To further characterize the FI2 in a new cohort of patients, we assessed the records of DM (n=31; 65 visits), PM (n=39; 61 visits), and IBM patients (n=58; 100 visits) No ceiling or floor effects were observed for individual FI2 components in PM or DM patients (Figure 1). In IBM, 54% of patients were unable to stand onto a step, resulting in a floor effect for this movement. Indeed, IBM patients had diminished step test endurance compared with PM patients, who in turn performed worse than DM patients (mean endurance score ± SEM, 2.8±0.4 vs. 3.6±0.5 and 5.8±0.5; all P<0.05). Conversely, IBM patients had better endurance performance than PM and DM patients in shoulder flexion (7.6±0.3 vs. 6.3±0.5 and 6.2±0.5; P<0.01) and shoulder abduction (6.6±0.4 vs. 5.4±0.5 and 5.0±0.5; P<0.01). Endurance scores in the hip flexion, head lift, toe lift, and heel lift tests did not significantly differ between groups. The composite FI2 score (mean of individual tests) was distributed similarly in DM, PM, and IBM (mean composite score ± SEM, 4.7±0.3, 4.2±0.3, and 4.6±0.3, respectively).
Figure 1.
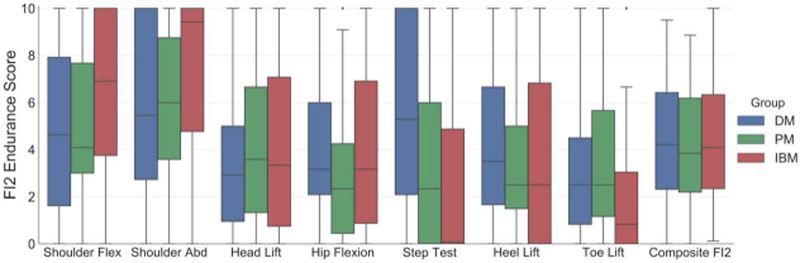
Distribution of individual and composite Functional Index-2 (FI2) endurance scores in dermatomyositis (DM; =31), polymyositis (PM; n=39), and inclusion body myositis (IBM; n=58). Data represented as a box-and-whisker plot, with boxes, whiskers, and single points demonstrating quartiles, upper and lower bounds, and outliers, respectively.
Comparison of FI2 and MMT in myositis patients
While IBM, DM, and PM patients did not significantly differ in overall FI2 scores, as measured by composite FI2, IBM patients had diminished maximal isometric muscle strength, measured by MMT (Figure 2). The FI2 had substantially higher variability between patients than did the MMT (interquartile range, 4.3 vs. 1.4, P<0.01). Overall and in each clinical group, FI2 correlated with MMT (Figure 2), although this correlation weakened at higher strength levels. In patients with normal or near-normal muscle strength (mean MMT >9.75; n=62), FI2 scores were typically abnormal (imperfect scores at 96.5% of patient-visits; mean composite FI2 score, 5.6/10) and highly variable (interquartile range, 3.3/10 to 7.8/10).
Figure 2.
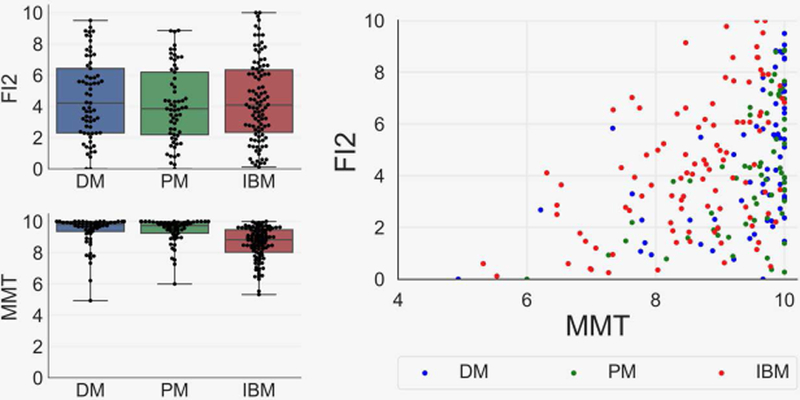
Composite Functional Index-2 (FI2) endurance and manual muscle testing (MMT) strength scores in myositis patients, and the association between FI2 and MMT. FI2 performance was comparable between groups, although inclusion body myositis (IBM; n=58) patients had lower MMT scores than dermatomyositis (DM; n=31) and polymyositis (PM; n-39) patients (mean MMT± SEM , 8.4± 0.1 vs. 9.5± 0.1 and 9.4±0.1, P<0.01). Positive linear correlations were observed between FI2 and MMT (r=0.43), although the correlation was weaker in patients with MMT scores greater than 9 (r=0.25).
Effect of covariates on FI2 scores
We next investigated whether FI2 score variation could be attributed to covariate factors. IBM patients were older than DM and PM patients (median age, 67 vs. 51 and 58 years; P<0.01). Age was not significantly associated with FI2 scores overall, in disease subgroups, or in the subset of patients with relatively normal muscle strength (generalized linear model, all P>0.05; Supplementary Figure 1). Serum CK levels were highest in PM patients compared with IBM and DM (median CK ± SEM, 629±200 vs. 376±67 and 81±55 units per liter). There was no clear relationship between serum CK levels and FI2 scores overall or in any patient subgroup (Supplementary Figure 2). Interstitial lung disease (ILD) was most common in DM patients compared with PM and IBM (prevalence, 29% vs. 10% and 0%). ILD was not associated with significant differences in FI2 overall or within disease subgroups (Supplementary Figure 3). These findings suggest that the FI2 was not modified substantially by covariate factors recorded in this study (i.e. age, serum CK, and presence of ILD).
Evolution of FI2 scores over time
Over time at the population level, FI2 and MMT scores tended to decrease over time while serum CK levels modestly increased (Figure 3). Using a mixed effect multilevel model to control for differing number of observations per patient, the association between FI2 and MMT scores was highly significant (P<0.001). Using the same model, MMT scores were inversely associated with CK levels (P<0.05). FI2 was inversely associated with CK over time but did not reach statistical significance (P>0.10). Together, these data suggest that FI2 and MMT scores evolve very similarly over time and tend to associate negatively with serum CK.
Figure 3.
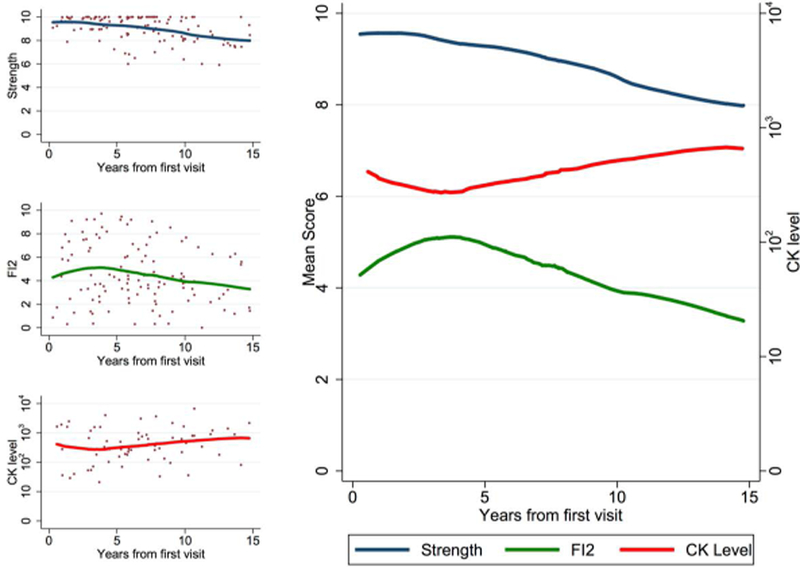
Manual muscle testing (MMT) and Functional Index-2 (FI2) scores evolved similarly over time, inversely to serum CK levels. Locally-weighted regression was performed on data from all myositis patients (n=128, 226 patient-visits).
A case of muscle impairment best described by FI2
Only one patient in our dataset had more than 4 visits with an FI2 evaluation, but her case was illustrative of the FI2’s utility. This patient was diagnosed with dermatomyositis and had a high baseline strength (composite MMT, 9.5) at the time of her first FI2 evaluation. After beginning corticosteroid therapy, her MMT improved immediately to normal levels. Her MMT then remained normal throughout her course of follow-up, despite fluctuations in CK and subjective reports of muscle impairment (disease “flares”). At follow-up visits, the FI2 detected changes in muscle endurance which were inversely proportionate to CK and corresponded with complaints of worsening muscle weakness and skin rash (Figure 4).
Figure 4.
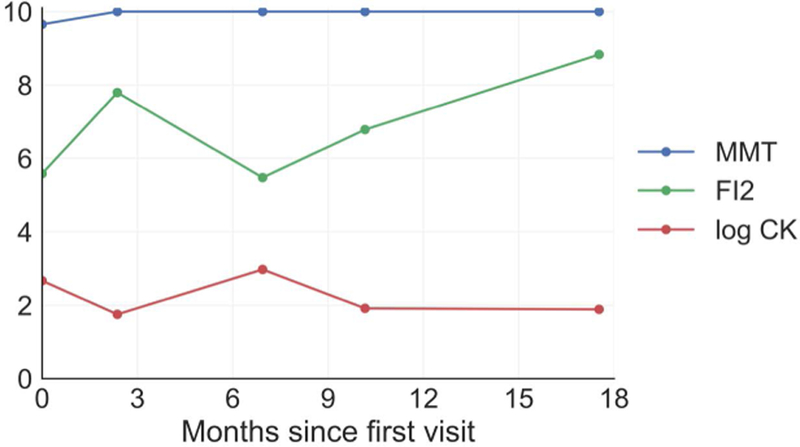
The evolution of strength, serum creatine kinase (CK), and Functional Index-2 (FI2) scores in a dermatomyositis patient with high baseline muscle strength. CK levels and FI2 evolved in an inverse pattern, with manual muscle testing (MMT) remaining at a “ceiling” score of 10. This patient complained of worsened muscle weakness and rash at month 7.
Comparison of truncated FI2 assessments
We next investigated potential methods to decrease the time requirements of FI2 testing (33 minutes for full examination). We first investigated the unilateral FI2 (21 minute time of administration), finding a high degree of symmetry in FI2 performance in all subgroups (Supplementary Figure 4). Next, we computed a subscore corresponding to each possible pair of individual FI2 movements and investigated their performance relative to the overall FI2. Several variable pair composites correlated very highly with the overall FI2 score. To rank these combinations by clinical utility, we generated a score that multiplies the correlation coefficient of the variable pair by the linear range rate of the pair (percentage of patients who did not score at the minimum or maximum). The shoulder abduction and hip flexion composite score ranked the highest by this metric (Supplementary Table 1). When performing the same analysis within clinical groups, results were largely the same, although the shoulder abduction and step test combination was ranked slightly higher in IBM. Figure 5 demonstrates the relationship between the shoulder abduction and hip flexion composite score and the overall FI2 composite score.
Figure 5.
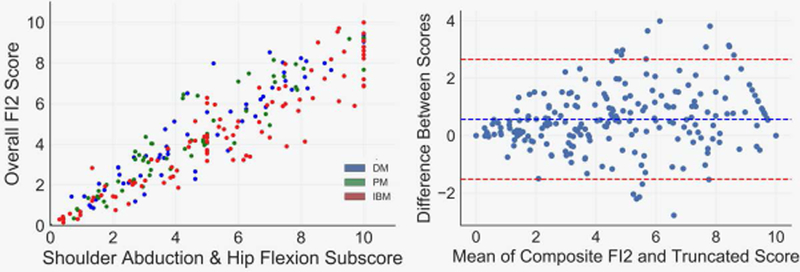
The shoulder abduction-hip flexion subscore has a broad linear range and is strongly associated with the overall Functional Index-2 (FI2) score. Data are demonstrated as a scatterplot and a Bland-Altman plot demonstrating high correlation and agreement (respectively) between the subscore and overall FI2 score.
DISCUSSION
Various neuromuscular diseases affect specific qualities of muscle functions differently (e.g. endurance vs. isometric vs. isokinetic qualities), and accurately measuring muscle function is a critical component in the care of patients with neuromuscular disease. In myositis, it is likely that disease stages play a differential role in muscle function; for example, isometric quality of muscle may be more affected by endomysial inflammation in the acute stage, with diminished endurance quality contributing more to daily activity limitations in chronically-affected patients 10. Varied complaints (e.g. fatigability vs. frank weakness) may illustrate this phenomenon. However, MMT measures maximal isometric torque generation and does not encompass other qualities (e.g. endurance) of muscle function. In this retrospective cohort study of DM, PM, and IBM patients, we characterized muscle function using both MMT and the FI2, a measure of dynamic muscle endurance.
Consistent with the data reported by Alexanderson et al., we did not observe any ceiling or floor effects for individual FI2 components in PM or DM patients 2. In IBM patients, the step test had a floor effect (median score 0/60), which is unsurprising given the preferential involvement of quadriceps and advanced age associated with IBM. Interestingly, IBM patients had better endurance than PM and DM in the shoulder abduction and flexion tasks, which may reflect the relative sparing of deltoids in IBM. However, IBM patients performed similarly to PM and DM patients in other movements, and the composite FI2 score in IBM was evenly and similarly distributed in all groups. Together, these data support the validity of the FI2 (including each subcomponent) in DM/PM and suggest that the FI2 is also useful in IBM, although some patients may benefit from a modified assessment of knee extension endurance in place of the normal stepping task.
Exploring the relationship between MMT and FI2, we observed that both tests were correlated and evolved similarly in all myositis subtypes, particularly in weaker patients. This likely reflects the demands of the FI2 at low strength levels, where the repetitive joint motion is limited more by force generation than by metabolic or “endurance” qualities. Indeed, the FI2 may have the greatest utility in measuring endurance quality of muscle in patients with relatively high MMT scores. Particularly in these patients with high strength, FI2 was much more variable than MMT, suggesting that a striking number of patients who were regarded as having normal muscle function do have measurable impairments. The reported case, detailing an inverse relationship between FI2 and disease flares despite consistently strong MMT scores, is consistent with our clinical experience and early data from a prospective cohort study 3 suggesting that the FI2 may be more responsive to disease activity than MMT. Should this observation be further established, endurance outcomes may have utility in the clinical trial setting, where subtle changes might be expected. It is worth noting that we do see patients who complain of muscle strength deterioration where their FI2 remains unchanged or improved; in such cases, their symptoms often resulted from joint or tendon injury and improved spontaneously or with physical rehabilitation.
Although our data imply a response of FI2 to changes in disease activity, prospective studies investigating changes in FI2, MMT, and muscle biopsy or imaging findings will be paramount in distinguishing which specific disease factors most affect different qualities of muscle function. Such studies are in progress in our clinic as well as others 3. Additionally, studies of the FI2 in other neuromuscular diseases (e.g. metabolic myopathies) may further reveal interesting dynamics, as our findings of disequilibrium between endurance and maximal strength are almost certainly not specific to myositis.
These data show the utility of the FI2 in identifying functional deficits in myositis patients, but the widespread clinical adoption of FI2 testing may be limited by the time requirements of its administration. While the validity of performing the FI2 unilaterally is supported by our data 22 minutes (the time taken if the bilateral movements are performed only unilaterally) is still time-intensive. For this reason, we explored our data to uncover two-subcomponent FI2 scores which can be completed in 10 minutes or less. The shoulder abduction and hip flexion composite score had the highest overall combination score of validity (correlation with overall FI2) and reliability (percentage of patients who did not attain minimum or maximum scores). However, several options emerged as viable, including the shoulder abduction and step test composite, which ranked highest in IBM patients. Clinicians may consider these data, along with disease classification and/or orthopedic limitations, to decide which approach may be most appropriate to monitor muscle endurance in their patients.
Our study has several limitations. Our patients had relatively high MMT scores, suggesting a potential bias in referral of stronger patients to have FI2 testing. Additionally, the use of Bohan and Peter for diagnosis of PM and DM likely produced heterogenous groups as compared with more recently established criteria. Limited data availability made it impossible to glean the full effect of different disease-modifying therapies (e.g. corticosteroids), comorbid conditions, or stage of disease on FI2 performance. Finally, although transformation of MMT data to a linear 10-point scale is a common method to quantify strength, MMT is truly an ordinal scale (i.e. scores are categorical, and not linear in nature). As such, some caution must be exercised when directly comparing variation in the MMT and FI2.
In this retrospective cohort study, we demonstrate a wide spectrum of muscle endurance deficits in patients with varying degrees of maximal isometric strength, including those with normal strength scores. Although prospective studies will provide clearer insight, our data suggest that isometric strength testing via MMT may be insufficient to completely describe muscle dysfunction in myositis patients. As such, clinicians may consider using the FI2 as an adjunct to MMT, particularly in patients with high baseline strength or complaints of fatigability. In conclusion, the FI2 measures an aspect of muscle function not well captured by MMT and may have utility in clinical care of myositis and other neuromuscular diseases.
Supplementary Material
Acknowledgements:
This work was financially supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. IPF is supported by a fellowship from The Myositis Association. TEL is supported by R01 NS082563 and NS094239. TC is supported by K12 5K12HD001097–19, Rehabilitation Medicine Scientist Program (RSMTP). LC-S is supported by the Huayi and Siuling Zhang Discovery Fund.
Abbreviations
- MMT
manual muscle testing
- FI2
Functional Index-2
- DM
dermatomyositis
- PM
polymyositis
- IBM
sporadic inclusion body myositis
- CK
serum creatine kinase
- LOWESS
locally-weighted scatterplot smoothing
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
References
- 1.Dalakas MC. Inflammatory Muscle Diseases. N Engl J Med 2015;373:393–394. [DOI] [PubMed] [Google Scholar]
- 2.Alexanderson H, Broman L, Tollback A, Josefson A, Lundberg IE, Stenstrom CH. Functional index-2: Validity and reliability of a disease-specific measure of impairment in patients with polymyositis and dermatomyositis. Arthritis Rheum 2006;55:114–122. [DOI] [PubMed] [Google Scholar]
- 3.Alexanderson H, Regardt M, Ottosson C, Alemo Munters L, Dastmalchi M, Dani L, Lundberg IE. Muscle Strength and Muscle Endurance During the First Year of Treatment of Polymyositis and Dermatomyositis: A Prospective Study. J Rheumatol 2018;45:538–546 [DOI] [PubMed] [Google Scholar]
- 4.Hanaoka BY, Cleary LC, Long DE, Srinivas A, Jenkins KA, Bush HM, Starnes CP, Rutledge M, Duan J, Fan Q, Fraser N, Crofford LJ. Physical impairment in patients with idiopathic inflammatory myopathies is associated with the American College of Rheumatology functional status measure. Clin Rheumatol 2015;34:1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexanderson H, Dastmalchi M, Esbjornsson-Liljedahl M, Opava CH, Lundberg IE. Benefits of intensive resistance training in patients with chronic polymyositis or dermatomyositis. Arthritis Rheum 2007;57:768–777. [DOI] [PubMed] [Google Scholar]
- 6.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–407. [DOI] [PubMed] [Google Scholar]
- 7.Rose MR, Group EIW. 188th ENMC International Workshop: Inclusion Body Myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord 2013;23:1044–1055. [DOI] [PubMed] [Google Scholar]
- 8.Rider LG, Werth VP, Huber AM, Alexanderson H, Rao AP, Ruperto N, Herbelin L, Barohn R, Isenberg D, Miller FW. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S118–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendall FP, McCreary EK, Provance PG. 1993. Muscles, Testing and Function : with Posture and Pain Williams & Wilkins: Baltimore, MD [Google Scholar]
- 10.Alemo Munters L, Dastmalchi M, Katz A, Esbjornsson M, Loell I, Hanna B, Liden M, Westerblad H, Lundberg IE, Alexanderson H. Improved exercise performance and increased aerobic capacity after endurance training of patients with stable polymyositis and dermatomyositis. Arthritis Res Ther 2013;15:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


