Abstract
The present study was performed to examine pressure transduction to the thoracic cavity during topical negative pressure (TNP) therapy of a sternotomy wound. Seven pigs underwent median sternotomy. Pressure transduction catheters were placed on the anterior surface of the heart (under the foam), in the pericardium (under the heart), in the left pleura and in the oesophagus at the level of the heart. The wound was sealed as for TNP therapy. The vacuum source was set to deliver negative pressures between −50 and −200 mmHg. The pressure on the anterior surface of the heart changed in a linear relationship with the applied negative pressure and was slightly lower than the applied negative pressure (−102 ± 9 mmHg at delivered −125 mmHg). Further down in the thoracic cavity, in the pericardium (under the heart), in the left pleura and in the oesophagus, the wound pressure was only slightly affected by TNP therapy. In conclusion during TNP therapy, negative pressure is effectively transmitted to anterior portions of the heart. This may explain our recent findings that TNP increases microvascular blood flow in the myocardium. The pressure difference between the anterior and the posterior portions of the heart causes the right ventricle to be sucked up towards the posterior parts of the sternum, where it might be exposed to the sharp edges of the sternal bone, which may result in heart injury.
Keywords: Experimental surgery, Mediastinal infection, Wound healing
Introduction
Cardiac surgery is complicated by poststernotomy mediastinitis in 1–5% of all procedures (1), and this is a potentially life‐threatening complication (2). The reported early mortality in poststernotomy mediastinitis following coronary bypass surgery grafting is between 8% and 25% 3, 4. Established treatment of poststernotomy mediastinitis includes surgical debridement, drainage, irrigation and reconstruction using pectoral muscle flap or omentum transposition. In 1999, Obdeijn et al. described a new mode of treatment for poststernotomy mediastinitis using a vacuum‐assisted closure technique (5), which is based on the principle of applying subatmospheric pressure by controlled suction to a sealed, airtight wound through a porous dressing. The technique, also known as topical negative pressure (TNP) therapy, has resulted in excellent clinical outcome 1, 2, 3, 4, 5. Scientific evidence regarding the mechanisms by which TNP promotes wound healing has started to emerge. The suction force created by TNP therapy enables the drainage of excessive fluid and debris, which leads to the removal of wound oedema, reduction in bacterial count and enhanced granulation tissue formation 6, 7, 8, 9. Knowledge of the effects of TNP in a sternotomy wound is limited (10).
It is known that blood flow to the muscle tissue of the wound edge is increased by TNP therapy 7, 8, 9. We recently show that TNP of a sternotomy wound stimulates blood flow to the myocardium (10). The present study was designed to examine to which extent negative pressure is transduced to the anterior portions of the heart and also to deeper parts of the thoracic cavity. A porcine sternotomy wound model was used, and pressure was recorded on the anterior portions of the heart, in the pericardium (behind the heart), in the left pleura and in the oesophagus during the application of TNP therapy at pressures between −50 and −200 mmHg.
Materials and methods
Animals
A porcine sternotomy wound model was used. Seven domestic landrace pigs with a mean weight of 70 kg were fasted overnight with free access to water. The study was approved by the Ethics Committee for Animal Research, Lund University, Sweden. The investigation complied with the ‘Guide for the Care and Use of Laboratory Animals’ as recommended by the US National Institutes of Health and published by the National Academies Press (1996). The anaesthesia was induced, and the surgical procedure was performed as previously described (11).
Anaesthesia and surgical preparation
An intramuscular injection of ketamine (Ketaminol vet™ 100 mg/ml; Farmaceutici Gellini S.p.A, Aprilia, Italy) 15 mg/kg body weight, in combination with midazolam (Dormicum 1 mg/ml; Roche, Stockholm, Sweden) and xylazine (Rompun vet.™ 20 mg/ml; Bayer AG, Leverkusen, Germany) 2 mg/kg, was used for premedication. Anaesthesia was induced by continuous intravenous infusion of propofol (Diprivan™ 20 mg/ml; AstraZeneca, Södertälje, Sweden) at a dosage of 0·1–0·2 mg/kg/minute in combination with intermittent fentanyl (Leptanal™; Lilly, France) and atracurium besylate (Tracrium™; Glaxo, Täby, Sweden) at doses of 0·02 μg/kg and 0·2–0·5 mg/kg, respectively. Before surgery, a tracheotomy (Portex endotracheal tube 7·5 mm internal diameter) was performed.
A ventilator (Servo‐Ventilator 900; Elema‐Schönander, Stockholm, Sweden) was used for mechanical ventilation. The same settings were used for all animals: volume‐controlled, pressure‐regulated ventilation, 8·5 l/minute, 15 breaths/minute and an inhaled oxygen fraction of 35%. A lower midline abdominal incision was made over the urinary bladder. The urinary bladder was exposed, and a urinary catheter (Silicone Foley Catheter; Tyco Healthcare, Tullamore, Ireland) was inserted, sutured and connected to a urinary bag (Unomedical a/s, Haarlev, Denmark). The abdominal incision was continuously sutured with Dermalon 2.0 (Davis‐Geck, Hampshire, UK).
A midline sternotomy was performed. The tip of a saline‐filled pressure catheter was placed in the left pleura, in the pericardium (under the heart) and on the surface of the heart (under foam) through the sternotomy wound. A fourth pressure transduction catheter was inserted into the oesophagus, through the mouth, so that the tip was positioned at the level of the heart. The pressure catheters were connected to a calibrated custom‐built pressure gauge. For probe positions, see Figure 1. A layer of polyurethane foam (KCI, Copenhagen, Denmark) was placed between the sternal edges. A second layer of foam was placed over the first layer and secured to the surrounding skin. The wound was sealed with a transparent adhesive drape (KCI), and the two evacuation tubes connected the foam with the vacuum source (V.A.C.® pump unit; KCI).
Figure 1.
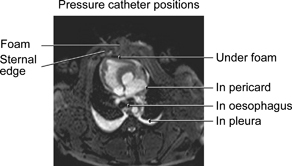
Magnetic resonance image illustrating a cross‐section of the pig thoracic cavity with an open sternotomy. First, the pressure transduction catheters were placed on the anterior surface of the heart (under the foam), in the pericardium (under the heart) and in the left pleura. A polyurethane foam dressing was placed between the sternal edges, and non collapsible drainage tubes were connected to the foam. The open wound was sealed with transparent adhesive drape, and drainage tubes are connected to a purpose‐built vacuum source. A fourth pressure transduction catheter was placed in the oesophagus at the level of the heart.
Experimental protocol
Wound pressures were recorded on the anterior surface of the heart (under the foam), in the pericardium (under the heart), in the left pleura and in the oesophagus. Wound pressures were measured both with the respirator on and with the respirator turned off at the end of inspiration. In order to eliminate systemic errors, both the sequence of applying negative pressure (−50, −75, −100, −125, −150, −175 and −200 mmHg) and the order in which the experiments were performed with the respirator turned on and off were varied between the animals in a randomised design.
Calculations and statistics
The experiments were performed on seven pigs. Calculations and statistical analysis were performed using GraphPad Prism® 4·0 software (GraphPad Software Inc, San Diego, CA). Statistical analysis was performed using Kruskal–Wallis test with Dunn’s test for multiple comparisons. Significance was defined as P < 0·05. Values are presented as means ± SEM.
Results
TNP was applied to the sternotomy wound at negative pressures between −50 and −200 mmHg. The pressure over the anterior surface of the heart (under the foam) changed in a linear relationship with the applied negative pressure and was slightly lower than the applied negative pressure (−102 ± 9 mmHg in the wound at delivered −125 mmHg; 2, 3). The pressure difference between the wound space and the vacuum source was larger at higher negative pressures (−39 ± 3 mmHg in the wound at delivered −50 mmHg and −163 ± 12 mmHg at delivered −40 mmHg).
Figure 2.
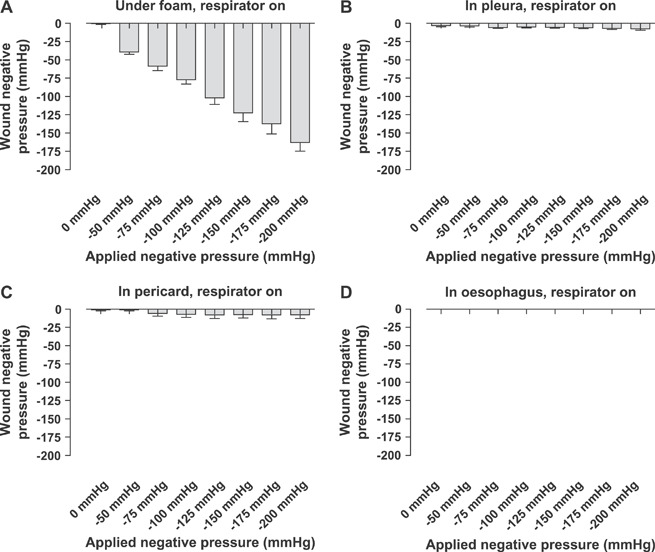
Wound pressures measured with the respirator on. The vacuum source was set to deliver negative pressures between −50 and −200 mmHg. Wound pressures were recorded using pressure transduction catheters on (A) the anterior surface of the heart (under the foam), (B) in the pericardium (under the heart), (C) in the left pleura and (D) in the oesophagus. Values are presented as means ± SEM from seven experiments. Note how the pressure under the foam changed in a linear relationship with the applied negative pressure, while the pressure further down in the thoracic cavity was not altered.
Figure 3.
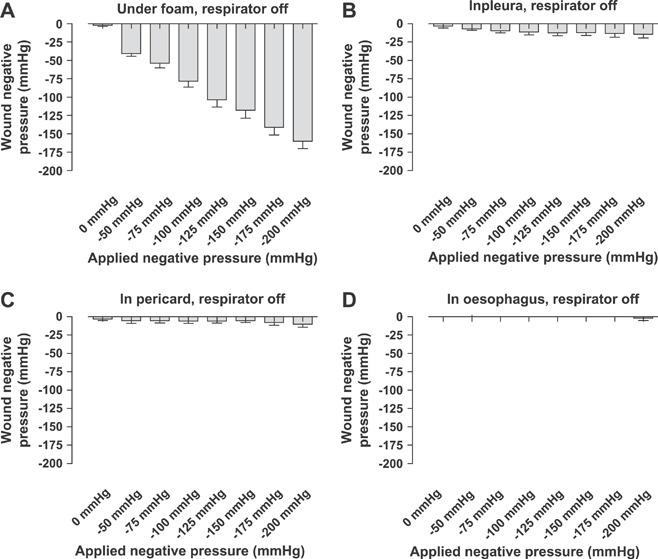
Wound pressures measured after the respirator had been turned off at the end of inspiration. The vacuum source was set to deliver negative pressures between −50 and −200 mmHg. Wound pressures were recorded using pressure transduction catheters on (A) the anterior surface of the heart (under the foam), (B) in the pericardium (under the heart), (C) in the left pleura and (D) in the oesophagus. Values are presented as means ± SEM from seven experiments. Note that the pressure recordings are similar to those with the respirator on.
Further down into the thoracic cavity, in the pericardium (under the heart), in the left pleura and in the oesophagus, the wound pressure was not significantly affected by TNP therapy (2, 3). The pressure recordings were similar with the respirator turned on as with the respirator turned off at the end of inspiration (2, 3).
Discussion
Mediastinitis is a devastating complication in open heart surgery. TNP is a recently introduced technique that promotes the healing of difficult wounds, including poststernotomy mediastinitis 4, 5, 8. The technique entails application of a negative pressure to a sealed, airtight wound. The suction force created by TNP therapy is known to stimulate blood flow to the wound edge and facilitate the drainage of excessive fluid and debris, which leads to the removal of wound oedema, reduction in bacterial count and enhanced granulation tissue formation 6, 7, 8, 9. Scientific evidence regarding of the effects in a sternotomy wound is still limited (10).
The present study was performed to explore to which extent negative pressure is transduced to the anterior portions of the heart and also to deeper locations in the sternotomy wound. We show that the pressure during TNP therapy is effectively delivered to the anterior portions of the heart. This may explain our recent findings that TNP at −50 mmHg increases microvascular blood flow in the myocardium (10). The blood flow effects by TNP are believed to be through mechanical stress and a pressure gradient across the tissue, which causes a surge of blood to the area 8, 9, 12, 13, 14. TNP is known to stimulate blood flow to the wound edge that is exposed to negative pressure, while tissue further from the vacuum source remains unaffected 8, 9. This is in accordance with the current findings that pressure is not transduced far beyond the locations of the open‐pore structure dressings of TNP therapy, for example to the pericardium (behind the heart), the pleura or the oesophagus.
The present results show negative pressure on the anterior portions of the heart, at similar levels as set on the vacuum source, while there is no negative pressure beneath the heart. This pressure difference may explain the finding that, upon the delivery of negative pressure, the anterior portion of the right ventricle is sucked up towards the anterior thoracic wall. The pressure causes the right ventricle to bulge into the space between the sternal edges, which deforms the anterior portion of the heart (15). We believe that this may have two negative effects.
First, pressure on the right ventricle may mechanically hinder venous return and cardiac pumping. Indeed, cardiac output and end diastolic volume are known to be slightly decreased upon application of negative pressure (15). Interestingly, interposition of four layers of paraffin gauze dressing over the heart during TNP therapy resulted in a lesser decrease in cardiac output (15). It is known that the interface dressings prevent the delivery of TNP (16).
Second, the pressure difference between the anterior and the posterior portions of the heart causes the right ventricle to be sucked up towards the posterior parts of the sternum where it might be exposed to the sharp edges of the sternal bone. This may result in right ventricle rupture, which is an uncommon but feared complication of TNP therapy in poststernotomy mediastinitis (17). Development of TNP therapy for sternotomy wounds by facilitating pressure transduction to the bottom of the wound may be beneficial in hindering the strong suction force on the heart. It is known that motion between the sternal edges in combination with adherent heart structures to the thoracic wall are factors that predispose for heart rupture. It is important to use surgical techniques to minimise these risk factors (18). In summary, adherences below the sternal edges must be released, and three or four layers of interface dressing should be placed over the anterior portions of the heart in order to cover and protect visible parts of the right ventricle from the sternal edges. The interface dressing reduces the formation of adherences between the sternum and the right ventricle, and the paraffin content facilitates movement.
In conclusion, the pressure over the anterior surface of the heart (under the foam) changed in a linear relationship with the applied negative pressure. These results may explain the positive effects on myocardial blood flow by TNP (10). Further down into the thoracic cavity, in the pericardium (under the heart), in the left pleura and in the oesophagus, the wound pressure was not affected by TNP therapy. The effect of negative pressure can therefore only be anticipated to be effective in superficial parts of the wound, for example on the sternotomy wound edges and on the anterior surface of the heart and not in deeper parts of the wound. The pressure difference between the anterior and the posterior portions of the heart during TNP therapy causes the right ventricle to be sucked up towards the posterior parts of the sternum where it might be exposed to the sharp edges of the sternal bone, which may result in heart injury.
Acknowledgements
We thank Martin Ugander, MD, PhD, for valuable contribution to the manuscript. This study was supported by the Anders Otto Swärds Foundation/Ulrika Eklunds Foundation, Anna Lisa and Sven Eric Lundgrens Foundation for Medical Research, Åke Wiberg Foundation, the M. Bergvall Foundation, the Swedish Medical Association, the Royal Physiographic Society in Lund, the Swedish Medical Research Council, the Crafoord Foundation, the Swedish Heart‐Lung Foundation, the Swedish Government Grant for Clinical Research and the Swedish Hypertension Society.
References
- 1. Raudat CW, Pagel J, Woodhall D, Wojtanowski M, Van Bergen R. Early intervention and aggressive management of infected median sternotomy incision: a review of 2242 open‐heart procedures. Am Surg 1997;63:238–41; discussion 241–2. [PubMed] [Google Scholar]
- 2. El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61:1030–6. [DOI] [PubMed] [Google Scholar]
- 3. Crabtree TD, Codd JE, Fraser VJ, Bailey MS, Olsen MA, Damiano RJ Jr. Multivariate analysis of risk factors for deep and superficial sternal infection after coronary artery bypass grafting at a tertiary care medical center. Semin Thorac Cardiovasc Surg 2004;16:53–61. [DOI] [PubMed] [Google Scholar]
- 4. Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid‐term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 2003;23:943–9. [DOI] [PubMed] [Google Scholar]
- 5. Obdeijn MC, De Lange MY, Lichtendahl DH, De Boer WJ. Vacuum‐assisted closure in the treatment of poststernotomy mediastinitis. Ann Thorac Surg 1999;68:2358–60. [DOI] [PubMed] [Google Scholar]
- 6. Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum‐assisted closure versus conventional treatment. Ann Thorac Surg 2005;79:2049–55. [DOI] [PubMed] [Google Scholar]
- 7. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 8. Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum‐assisted closure therapy. Ann Thorac Surg 2005;79:1724–30; discussion 1730–1. [DOI] [PubMed] [Google Scholar]
- 9. Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 2004;12:600–6. [DOI] [PubMed] [Google Scholar]
- 10. Lindstedt S, Malmsjö M, Ingemansson R. Blood flow changes in normal and ischemic myocardium during topically applied negative pressure. Ann Thor Surg 2007;84:568–573. [DOI] [PubMed] [Google Scholar]
- 11. Petzina R, Gustafsson L, Mokhtari A, Ingemansson R, Malmsjo M. Effect of vacuum‐assisted closure on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur J Cardiothorac Surg 2006;30:85–9. [DOI] [PubMed] [Google Scholar]
- 12. Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum‐assisted closure on wound microcirculation: an experimental study. Asian J Surg 2005;28:211–7. [DOI] [PubMed] [Google Scholar]
- 13. Fabian TS, Kaufman HJ, Lett ED, Thomas JB, Rawl DK, Lewis PL, Summitt JB, Merryman JI, Schaeffer TD, Sargent LA, Burns RP. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full‐thickness wound healing. Am Surg 2000;66:1136–43. [PubMed] [Google Scholar]
- 14. Fleck TM, Fleck M, Moidl R, Czerny M, Koller R, Giovanoli P, Hiesmayer MJ, Zimpfer D, Wolner E, Grabenwoger M. The vacuum‐assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thorac Surg 2002;74:1596–600; discussion 1600. [DOI] [PubMed] [Google Scholar]
- 15. Petzina R, Ugander M, Gustafsson L, Engblom H, Sjögren J, Hetzer R, Ingemansson R, Arheden H, Malmsjö M. Hemodynamic effects of vacuum‐assisted closure therapy in cardiac surgery, assessment using magnetic resonance imaging. J Thorac Cardiothorac Surg 2007;133:1154–1162. [DOI] [PubMed] [Google Scholar]
- 16. Jones SM, Banwell PE, Shakespeare PG. Interface dressings influence the delivery of topical negative‐pressure therapy. Plast Reconstr Surg 2005;116:1023–8. [DOI] [PubMed] [Google Scholar]
- 17. Abu‐Omar Y, Naik MJ, Catarino PA, Ratnatunga C. Right ventricular rupture during use of high‐pressure suction drainage in the management of poststernotomy mediastinitis. Ann Thorac Surg 2003;76:974; author reply 974–5. [DOI] [PubMed] [Google Scholar]
- 18. Gustafsson RI, Sjogren J, Ingemansson R. Deep sternal wound infection: a sternal‐sparing technique with vacuum‐assisted closure therapy. Ann Thorac Surg 2003;76:2048–53; discussion 2053. [DOI] [PubMed] [Google Scholar]


