Figure 1. The uptake and transport of low-density lipoprotein (LDL) in endothelial cells (ECs).
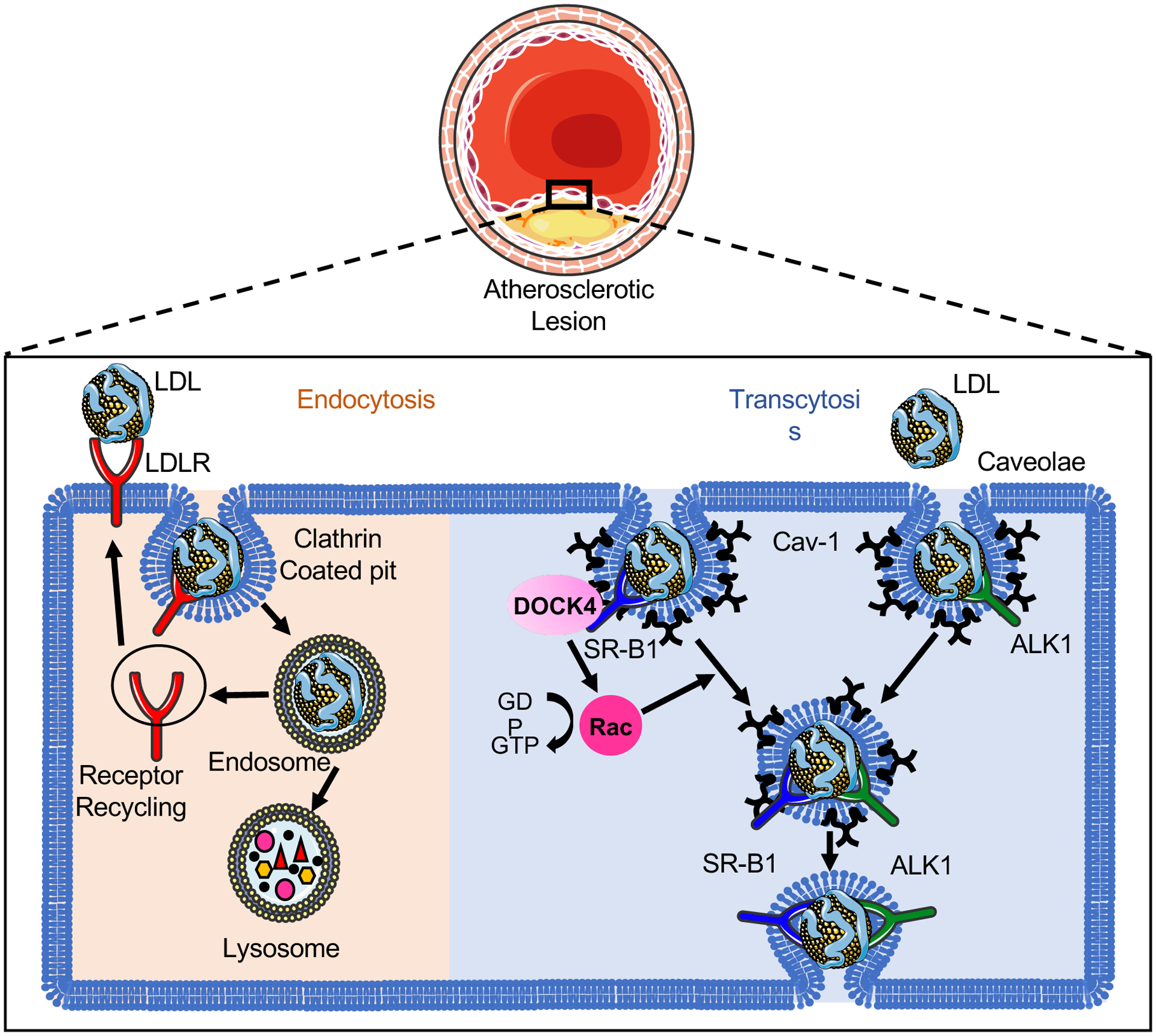
The classical LDL receptor (LDLR) pathway mediates the clustering of LDL-LDLR complex into clathrin-coated pits. The internalized coated vehicle fuses with endosomes, where the low pH in the endosome leads to the dissociation of LDLR from LDL and the recycling to the cell surface. The retained LDL particles travel further to lysosome and subsequently undergoes lysosomal degradation. Unlike the LDLR–dependent endocytosis pathway through which LDL is degraded by lysosomal enzymes releasing free cholesterol, intact LDL can transport across the endothelium by specific transcytosis pathways involved in caveolae, activin-like kinase 1 (ALK1) or scavenger receptor B1 (SR-B1) protein. Caveolin-1 (Cav-1) is the major protein component of caveolae that is required for the formation of caveolae in ECs. SR-B1 and ALK1 directly bind the LDL and locate in the caveolae. The recruitment of the guanine-nucleotide-exchange factor DOCK4 to SR-B1 facilitates the SR-B1 internalization and LDL transcytosis by coupling LDL binding to SR-B1 with Rac1 activation. The endocytosed LDL particles are then transferred to the opposite side of the cell leading to the retention of LDL in the arterial wall.
