Figure 2. Low barrier hydrogen bonds in catalytic triads.
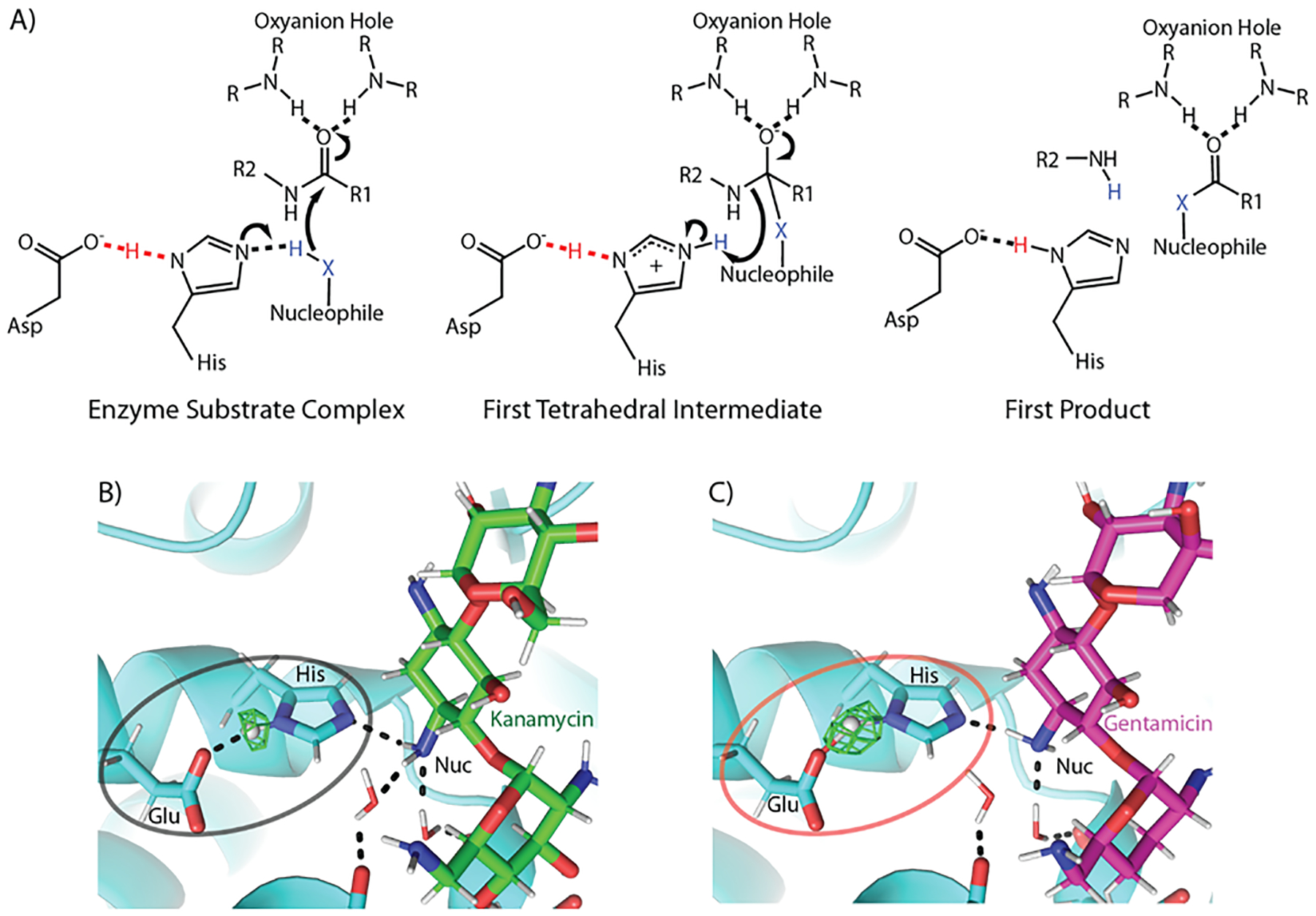
A) Three states of a general catalytic triad mechanism are shown. In the starting enzyme substrate complex, a hydrogen bond exists between the aspartic acid and the histidine Nδ, an interaction found in some instances to be an LBHB, increases the basicity of the histidine Nε (left). This permits the abstraction of a proton from the nucleophile, which subsequently attacks the peptide bond and generates the first tetrahedral intermediate (center). The first intermediate collapses resulting in the first two products of the reaction (right). R groups indicate continuation of the protein chain. B) The local chemical environment of the non-canonical (Glu-His-antibiotic amine) catalytic triad found in an aminoglycoside acetyltransferase dictates the type of hydrogen bond when bound to B) kanamycin and C) gentamicin. The Fo-Fc nuclear omit density for the hydrogen atom involved in the hydrogen bond between the catalytic residues is shown in green. For the least catalytically preferred substrate (kanamycin), a canonical hydrogen bond is found, whereas for one of the best turned over antibiotics (gentamicin) a low barrier hydrogen bond is found.
