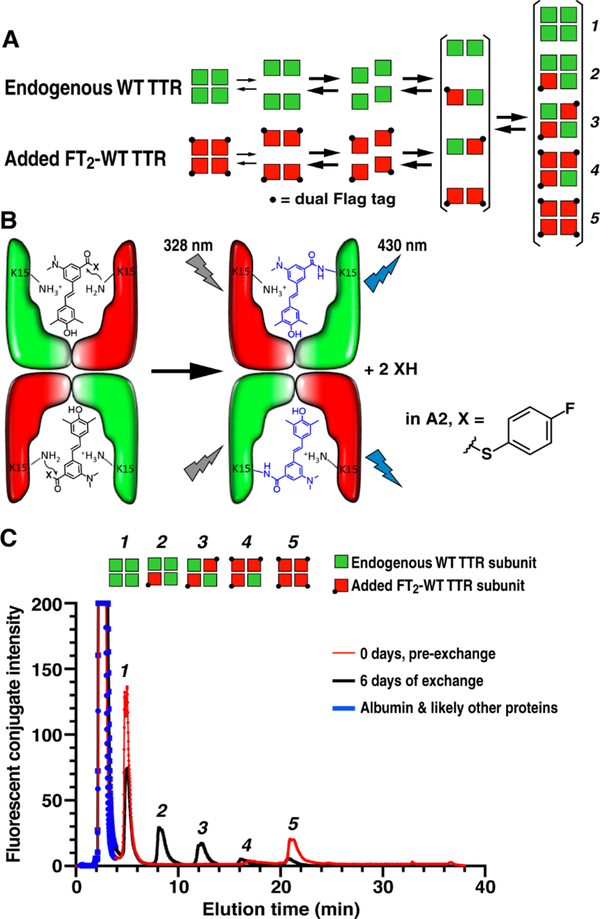
Figure 1. Subunit Exchange to Quantify TTR Tetramer Kinetic Stability.
(A) Schematic of the steps involved in subunit exchange between endogenous TTR in human plasma represented by green subunits and added dual-FLAG tagged WT TTR (FT2-WT TTR) depicted by red subunits. Rate-limiting tetramer dissociation depleting homotetramers 1 and 5, and subunit re-association to afford heterotetramers 2, 3 and 4 allow the apparent rate constants for subunit exchange (kex) to be determined. (B) Aliquots removed as a function of time are removed from the subunit exchange reaction and injected into an excess of A2, which binds to the two binding sites in TTR and then reacts with the Lys-15 residues, rendering the TTR conjugates detectable by fluorescence in the background of the 4000+ proteins comprising plasma. (C) Ion exchange chromatography of whole human plasma with fluorescence detection can be used to follow the abundances of tetramers 1-5 as a function of time, yielding the time courses in Figure 2 and Supplemental Figures S1 and S2, which can be fit to determine kex as a function of the concentration of various kinetic stabilizers.