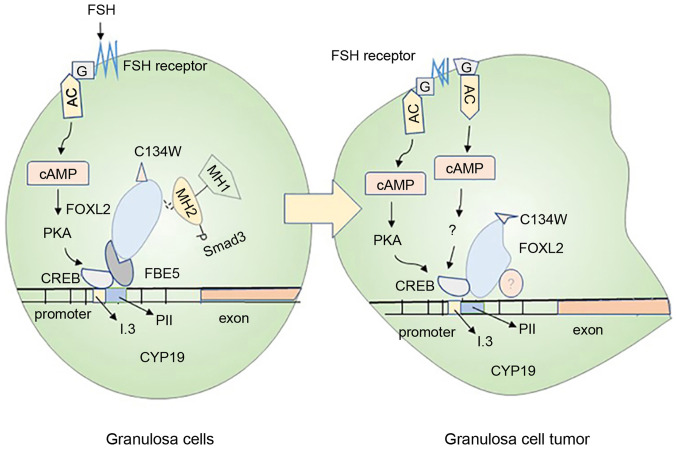
Figure 3.
Mechanisms of ovarian granulosa cell carcinogenesis. The normal granulosa cells shown in the left panel and the cancerous granulosa cells shown in the right panel mainly introduce the carcinogenesis mechanism of the cells. In normal granulosa cells, FSH binds to the receptor and acts through the trimer G protein (G) and adenylate cyclase, then, combine to the CRE binding site of CYP19A1 through the protein kinase A pathway, causing increased expression of aromatase. In the granulosa cells on the right, the FSH may not depend on the activation of this pathway, and the cancerization mainly originates from the mutation of the trimer G protein (G) and the activation of the FSH receptor. In addition, Smad3 in normal granulosa cells cooperates with FOXL2:C134W and FBE5 to act on CYP19A1 gene. The mutant FOXL2:C134W in granulosa cell tumors binds to another specific site of CYP19 and recruits unknown proteins, causing cell mutations. These are likely to cause abnormal expression of aromatase in granulosa cells and cancerization of cells. FSH, follicle-stimulating hormone; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; CREB, cAMP-response element binding protein.