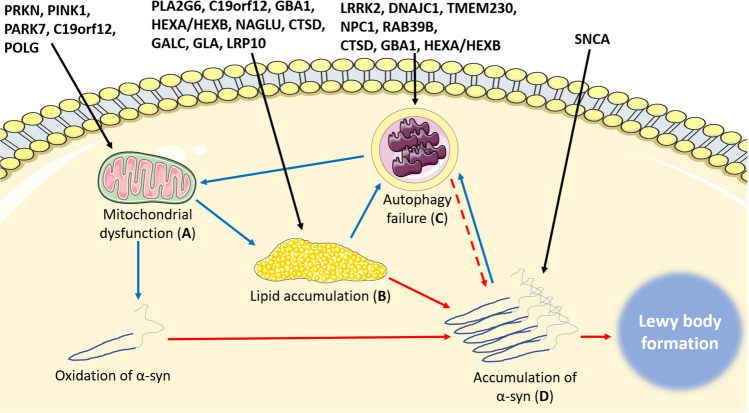
Fig. 4.
Multiple pathways leading to Lewy body formation. a Mutations causing mitochondrial dysfunction may contribute to Lewy body formation by increasing oxidation of α-synuclein, leading to aggregation and eventual formation of a Lewy body. b Mutations in genes encoding lipid-degrading enzymes such as GALC, GBA and CATD can directly lead to increased levels of lipid substrates known to be permissive to α-synuclein aggregation, including psychosine, glucosylsphingosine and heparan sulphate, respectively. Alternatively, mitochondrial dysfunction (a) leads to increased abundance of lipid droplets known to facilitate α-synuclein aggregation. Elevated levels of lipids are likely to overwhelm autophagic mechanisms within neurons, leading to autophagy failure after sustained elevations in lipid species (c). c Autophagy failure induces mitochondrial dysfunction (a) by impeding mitochondrial quality control by reductions in mitophagy, and potentially also leads to α-synuclein aggregation by reduced turnover (dashes). (d) Accumulation of α-synuclein may occur directly due to disassembley of tetramers into aggregation-prone monomers or increased abundance of α-synuclein protein, or indirectly through (a), (b), or (c). Increased accumulation of α-synuclein over time leads to assembly into Lewy bodies. Black lines indicate the mechanism directly affected by specific mutations, blue lines indicate indirect influences on α-synuclein aggregation through interactions between mechanisms, and red lines indicate direct influences on α-synuclein aggregation