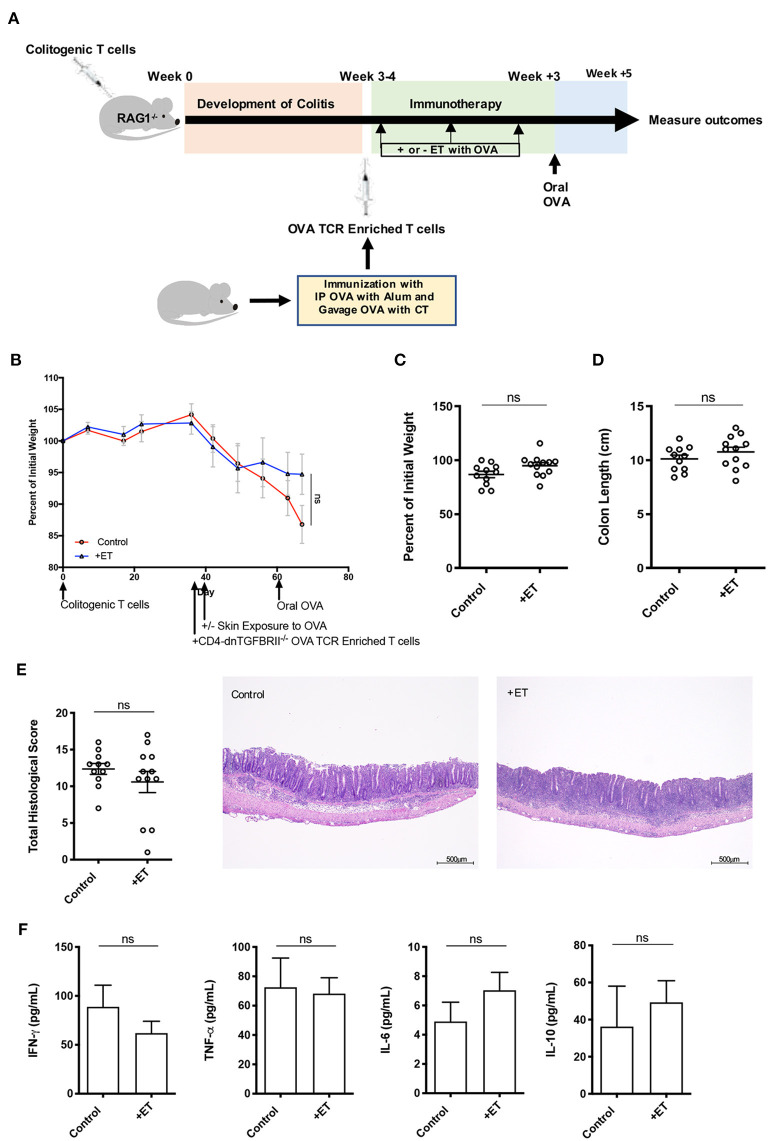
Figure 4.
TGF-β was necessary for ET to abrogate colitis. (A) Schematic demonstrating the design of the experiments: ET, Epicutaneous immunotherapy. RAG1−/− mice were injected with colitogenic T cells (CD4+CD45RBhi) from wild-type mice. Once mice exhibited symptoms (weight loss, loose stool, or blood in the stool) of colitis at week 3 or 4, mice were injected with OVA TCR enriched T cells from CD4-dnTGFBRII mice that were immunized with IP OVA with alum followed by gavage feeding with OVA with cholera toxin (CT). After this injection, mice were exposed on the skin (+ET) with Viaskin containing OVA or vehicle alone (-ET) weekly for 3 weeks. All mice then received an oral dose of OVA given by gavage. (B) The percentage of initial body weight of mice with colitis induced via the CD4+CD45RBhi transfer with the addition of OVA TCR enriched T cells from CD4-dnTGFBRII mice and exposed to OVA-Viaskin (+ET) or not (control). (C) The final percentage of initial body weight as measured when sacrificing them. (D) Colon length of the mice after sacrificing them. (E) Histological score of colon samples as determined by a pathologist blinded to the treatment group. Representative H&E sections of colon at 40× magnification that demonstrate both control and treated groups (control and +ET) with erosions, loss of crypts, an expansion of the submucosa and infiltration of the muscularis by inflammatory cells. The total histological score of the representative section of control and +ET were 13 and 14, respectively. (F) Cytokine production by cultured colon samples (3 pooled experiments of 4–5 mice/group; ns, not significant).