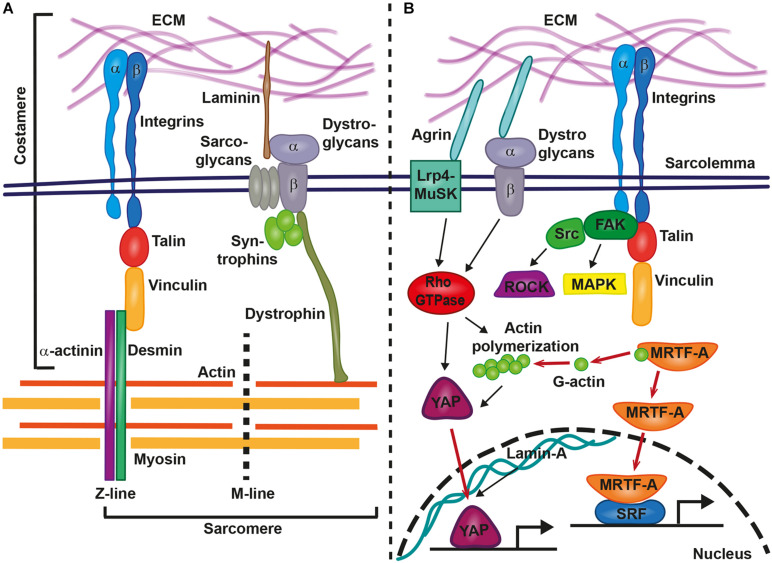
FIGURE 1.
Involvement of costameres in mechanotransduction in cardiomyocytes. Costameres are composed of structural and signaling proteins. These structures provide the link between extracellular matrix (ECM) and the sarcomere (A) or result in the activation of various signaling cascades, that lead to the translocation of transcription factors to the nucleus and gene transcription changes (B). Inside-out and outside-in signaling is mediated by integrins and the structural proteins talin, vinculin, α-actinin, and desmin provide the linkage from the sarcolemma to the sarcomere (actin, myosin, M-line, and Z-line). Similarly, The ECM-bound laminin connects via the dystrophin-glycoprotein complex with sarcomeric cytoskeletal components. Agrin acts via the Lrp4-MuSK or the dystrophin-glycoprotein complex on intracellular signaling cascades. Integrins recruit the focal adhesion kinase (FAK)-steroid receptor coactivator (Src)-complex, Rho-associated coiled-coil containing protein kinases (ROCKs) and mitogen-activated protein kinases (MAPKs). Rho-GTPase-induced actin polymerization liberates myocardin-related transcription factor A (MRTF-A) for nuclear translocation and supports the transport of YAP into the nucleus. Other relevant signaling cascades and proteins are described in the main text.