Abstract
Background
Difficulties are often encountered while controlling atrial fibrillation (AF), especially in hemodialysis (HD) patients. Previous data revealed that cryoballoon ablation (CBA) for treating paroxysmal atrial fibrillation (PAF) was not inferior to radiofrequency ablation (RFA); however, HD patients were excluded in this prior trial. Thus, the efficacy of CBA for HD patients is still unknown.
Methods
This retrospective study analyzed HD patients who underwent catheter ablation (CA) for AF from August 2011 to June 2019. Patients who received CBA (CBA group) and those who received RFA (RFA group) were compared. The primary endpoint was defined as freedom from a composite outcome (a documented recurrence of any atrial tachyarrhythmia or a prescription of antiarrhythmic drugs) at one year after CA.
Results
The RFA and CBA groups were composed of 21 and 23 patients, respectively. Freedom from a composite outcome was 58.4% in the RFA group and 68.2% in the CBA group (Log-rank: p = 0.571).
Conclusion
Our results suggest that patients on HD with AF who were treated with CBA tended to have better outcomes than patients treated with RFA. Therefore, CBA could be a suitable ablation method for HD patients.
Keywords: Hemodialysis, Cryoballoon ablation, Radiofrequency ablation, Atrial fibrillation, Catheter ablation
1. Introduction
Atrial fibrillation (AF) is observed in 12% of patients who start hemodialysis (HD), and 12% of patients with a normal sinus rhythm develop AF within 2 years [1]. HD patients with AF exhibit an much higher mortality rate than those without AF [1]. Further, over 30% of HD patients aged >70 years have AF [2]. We sometimes encounter the necessity of maintaining sinus rhythm because of symptoms, such as palpitation, despite sufficient heart rate control.
Literature about catheter ablation (CA) for AF in HD patients are limited. A previous study reported on radiofrequency catheter ablation (RFA) for paroxysmal atrial fibrillation (PAF), but found that the outcome was worse in comparison to non-HD patients [3]. The FIRE and ICE trial revealed the efficacy of cryoballoon ablation (CBA) [4], but HD patients were excluded from this trial. Thus, the efficacy of CBA for HD patients is still unknown. In the present study, we aimed to compare the efficacy of CBA for AF with RFA.
2. Methods
We enrolled 44 consecutive HD patients who underwent CA for AF in Shonan Kamakura General Hospital from August 2011 to May 2019. The study protocol was approved by Mirai Medical Center Ethical Committee (TGE01468-024), and the study was conducted according to the tenets of the Declaration of Helsinki. This study enrolled not only patients with PAF but also patients with persistent atrial fibrillation (PeAF) and long-standing persistent atrial fibrillation (LSAF).
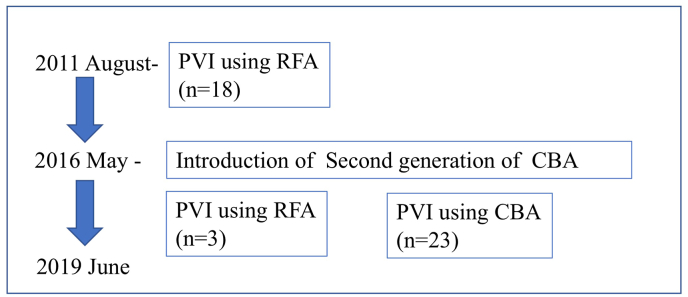
The selection of CA method was based on the timing of the introduction of the CBA and Pulmonary vein(PV) anatomies. Before the introduction of the CBA, all patients underwent pulmonary vein isolation (PVI) using RFA. After the introduction of the CBA in 2016, 23 patients underwent PVI with CBA, while three patients underwent PVI with RFA because of a large PV (Fig. 1).
Fig. 1.
Flowchart of the study population.
CBA cryoballoon ablation, HD hemodialysis, PVI pulmonary vein isolation, RFA radiofrequency ablation.
In this study, we compared patients who received CBA (CBA group) and those who received RFA (RFA group). The primary endpoint was defined as freedom from a composite outcome of a documented recurrence of atrial tachyarrhythmia (ATA) (lasting more than 30 s) or a prescription of an AAD (class I or III). This primary endpoint was analyzed at one year after the first CA. Episodes of ATA within the first 3-month after the procedure were not considered instances of recurrence (blanking period). The use of AAD after blanking period was based on the physician’s discretion considering to patient’s symptoms such as palpitation with or without a documented ATA. Within the blanking period, recurrent arrhythmias could be managed with AAD and cardioversion without penalty with regard to the primary end point. The procedure time and complications were also evaluated. The complications were defined as unexpected events which required intervention or prolonged hospital stays beyond the scheduled period.
All AADs were discontinued for at least three days before CA. All patients received warfarin for at least three weeks, and the internationalized normalized ratio for blood coagulation was checked before ablation. A nasogastric thermometer (Esophastar, JAPAN LIFELINE, Japan) was inserted to measure the esophageal temperature during ablation. All procedures during ablation were performed under sedation with intravenous propofol.
An activated clotting time of 300–350 s was maintained with a continuous infusion of heparin during the procedure. Isoproterenol (5–10 μg) was injected intravenously after the PVI. If sustained or non-sustained AF was reproducibly induced from non-PV foci, they were additionally ablated [5]. When non-PV foci were located in the superior vena cava (SVC), the SVC was electrically isolated [6]
Dormant PV conduction was intended to be induced with adenosine triphosphate (20 mg) under isoproterenol infusion. If dormant PV conduction was captured, an additional CBA or touch-up ablation was performed.
2.1. RFA
After performing a transseptal puncture using a one-puncture technique, two long sheaths were inserted into the left atrium (LA) [7]. A pulmonary venography was performed to determine the anatomical relationships of the PV ostia and the LA. Circular mapping catheters were placed in the superior or inferior PVs, and ipsilateral PVs were circumferentially and extensively ablated under fluoroscopic and electrophysiological guidance. RFA applications were delivered using either an 8-mm-tip ablation catheter (Japan Lifeline Inc, Japan), SmartTouch ThermoCool® Surround Flow irrigated-tip ablation catheter (Biosense Webster, Irvine, CA), or TactiCath™ Quartz (Abbott, Chicago, IL). The maximum power applied on the LA posterior wall was 20–30 W and that at the anterior aspect of the PVs was 30–40 W [8]. The RFA energy was delivered for 30–40 s at each site. When the CARTO system (Biosense Webster, Irvine, CA) and contact force catheter were used, RFA was delivered until the ablation index reached >400 at the posterior wall/roof and the ablation index was >450 at the anterior wall. A point-by-point RFA was performed. When the EnSite system (Abbott, Chicago, IL) was used, the lesion size index approach was conducted [9]; RFA was delivered until the lesion size index was >5 at the posterior wall/roof and the lesion size index was >5.5 at the anterior wall. The esophageal temperature has been routinely measured during RFA applications to avoid esophagus-related complications [10]. In cases with an esophageal temperature of >39 °C, RFA was discontinued.
2.2. CBA
All procedures were performed under deep sedation with propofol. A 15-Fr steerable sheath (FlexCath Advance; Medtronic, Ireland) was introduced into the LA, and an inner lumen mapping catheter (Achieve; Medtronic, Ireland) was sequentially positioned in each PV to obtain the baseline PV potential. A 28-mm CBA (Arctic Front Advance or Arctic Front Advance PRO, Medtronic, Ireland) was advanced over the inner lumen mapping catheter up to the LA, inflated, and positioned in the PV ostium of each vein. The CBA was inflated and advanced to the ostium of each PV in an attempt to obtain complete occlusion. After verifying the complete occlusion of the PV ostium, cryoenergy was applied. After documentation of the PVI, the freeze cycle was extended for another 120 s. If no real-time PV signal recording could be obtained, a standard freeze cycle of 180 s was applied [11]. In cases with either a minimum temperature of ≤ −60 °C, an esophageal temperature of <18.5 °C, or the PV potential remained after a 100 s application of cryoenergy, CBA was discontinued [12]. If the PV potential did not disappear after several applications at the same PV, touch-up ablation with a 4-mm-tip irrigated ablation catheter (FlexAbility™ Ablation Catheter, Abbott, Chicago, IL) was performed. To avoid phrenic nerve injury, CBA applications for the right PVs were performed under monitoring of the diaphragmatic compound motor action potentials during phrenic nerve pacing [13]
2.3. Follow-up
Anticoagulation was discontinued three months after the index procedure. All patients were followed at one, three, six, and 12 months after the ablation procedure. Moreover, 12-lead electrocardiography (ECG) was performed at every visit, and 24 h Holter ECG monitoring was performed at three months and 12 months after ablation. If patients had a symptom event even if no AF was documented in the Holter ECG, portal ECG monitoring (hcg-801 heartscan, Omron, Japan) was used.
2.4. Statistical analysis
Data are expressed as means ± standard deviations for continuous variables and as frequencies and percentages for categorical variables. To compare the two groups, chi-squared analysis or Fisher’s exact test was used for categorical variables and an unpaired t-test or Wilcoxon analysis was used for continuous variables. The follow-up period was calculated from the date of the procedure to the date of primary endpoint or censoring.
The Kaplan-Meier method was used to analyze any unadjusted ATA recurrence rates, which were compared using the log-rank test. A two-side p value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 26.0 (IBM Inc., Armonk, NY, USA).
3. Results
3.1. Baseline characteristics
Among the 44 patients who received CA for AF, 21 patients (47.7%) received RFA and 23 patients (52.3%) received CBA. Diabetes mellitus and dyslipidemia were statistically lower in the RFA group and the patients in the CBA group were of older age (Table 1).
Table 1.
Baseline demographics.
| RFA group(n = 21) | CBA group(n = 23) | p | |
|---|---|---|---|
| PeAF, LSAF, n(%) | 4(19.0) | 6(26.1) | 0.578 |
| Age, year(±SD) | 64.9(±6.8) | 70.7(±8.4) | 0.017 |
| Male, n(%) | 15(71.4) | 15(65.2) | 0.659 |
| Height, cm (±SD) | 162.8(±6.4) | 163.7(±8.8) | 0.708 |
| Weight, kg (±SD) | 56.2(±9.9) | 59.2(±12.6) | 0.389 |
| BMI(±SD) | 21.2(±3.6) | 22.0(±3.8) | 0.481 |
| Hypertension, n(%) | 18(85.7) | 18(78.3) | 0.522 |
| Dyslipidemia, n(%) | 4(19.0) | 11(47.8) | 0.044 |
| DM, n(%) | 1(4.8) | 8(34.8) | 0.014 |
| Insulin, n(%) | 0(0) | 4(17.4) | 0.045 |
| Prior CHF, n(%) | 3(14.3) | 6(26.1) | 0.332 |
| 65–74 years old n(%) | 12(57.1) | 12(52.2) | 0.741 |
| over 75 years old, n(%) | 1(4.8) | 7(30.4) | 0.027 |
| Stroke, n(%) | 4(19.0) | 1(4.3) | 0.125 |
| Vascular disease, n(%) | 4(19.0) | 4(17.4) | 0.230 |
| ACE-I, n(%) | 8(38.1) | 8(34.8) | 0.820 |
| β blocker, n(%) | 10(47.6) | 16(69.6) | 0.139 |
| HD Duration(year)(±SD) | 10.9(±10.0) | 10.4(±10.1) | 0.859 |
| AF year (±SD) | 2.3(±2.1) | 1.7(±1.3) | 0.307 |
| CHADS2 score (±SD) | 1.6(±1.0) | 1.7(±1.0) | 0.675 |
| CHA2DS2-VASc (±SD) | 2.6(±1.4) | 3.1(±1.3) | 0.175 |
| LAD(mm) (±SD) | 42.4(±6.7) | 43.9(±6.1) | 0.458 |
| LVDd(mm)(±SD) | 49.6(±5.3) | 50.1(±8.5) | 0.793 |
| EF(%) (±SD) | 54.1 (±12.4) | 54.5(±15.1) | 0.239 |
| IVSth(mm) (±SD) | 11.2(±1.5) | 11.0(±1.1) | 0.612 |
| LVPWth(mm) (±SD) | 11.3(±1.1) | 10.1(±2.0) | 0.429 |
PeAF: persistent atrial fibrillation, LSAF: long-standing persistent atrial fibrillation, SD: standard deviation, RFA radiofrequency ablation, CBA cryoballoon ablation, BMI: body mass index, DM: diabetes mellitus, CHF: congestive heart failure, EF: ejection fraction, HD: hemodialysis, AF: atrial fibrillation, LAD: left atrial diameter, LVDd: left ventricular end-diastolic diameter, IVSth: interventricular septal wall thickness, LVPWth left ventricular posterior wall thickness.
3.2. Procedural characteristics of CA
The mean duration of the procedure was 179.4 ± 51.9 min in the RFA group and 96.7 ± 36.9 min in the CBA group (p < 0.001). The mean duration of the total fluoroscopic time was 60.1 ± 41.2 min in the RFA group and 26.9 ± 12.8 min in the CBA group (p = 0.001). Regarding the procedures, 20 out of 21 patients in the RFA group (95.2%) received cavo-tricuspid isthmus linear ablation (CTI), whereas none of the patients in the CBA group underwent a CTI (p < 0.001) (Table 2). In the RFA group, four of 21 patients (19.0%) underwent RFA using a contact force catheter. Five out of 23 patients (21.7%) received touch-up ablation in the CBA group.
Table 2.
Procedure characteristics.
| RFA group(n = 21) | CBA group(n = 23) | p | |
|---|---|---|---|
| CTI linear ablation, n(%) | 20(95.2) | 0(0) | <0.001 |
| Non-PVI, n(%) | 4(19.1) | 1(4.3) | 0.125 |
| SVC isolation, n(%) | 3(14.2) | 0(0) | 0.060 |
| Procedure time, min | 179.4(±51.9) | 96.7(±36.9) | <0.001 |
| fluoroscopic time, min | 60.1(±41.2) | 26.9(±12.8) | 0.001 |
RFA radiofrequency ablation, CBA cryoballoon ablation, CTI: cavo-tricuspid isthmus, non-PVI: non-pulmonary vein isolation, SVC: superior vena cava.
3.3. Primary endpoint
Two patients in the RFA group were lost to follow-up. Two patients died within one year, and these two patients belonged to the RFA group. One patient died of respiratory failure in another hospital, and the other patient died of sepsis. Only one of these two patients had an AF recurrence before death.
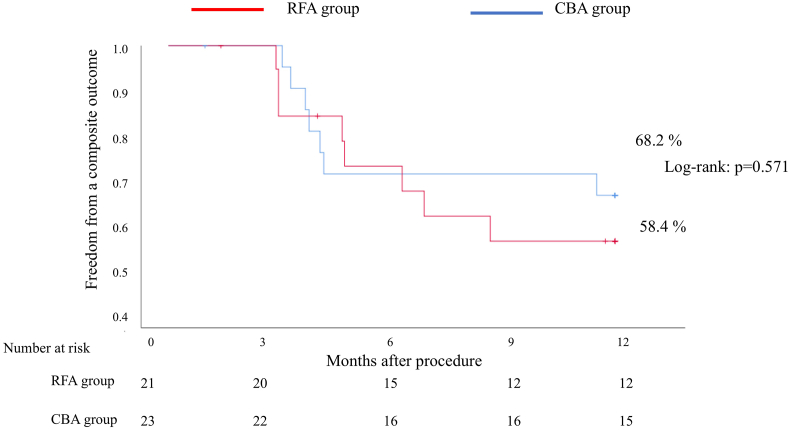
In the RFA group, eight out of 21 patients had a composite outcome of ATA or use of an AAD (7 patients with ATA and 1 patient with AAD). In the CBA group, seven out of 23 patients had a composite outcome of ATA or use of an AAD (2 patients with ATA and 5 patients with AAD). For the primary endpoint, freedom from a composite outcome of a documented recurrence of any ATA or a prescription of an AAD at one year was 58.4% in the RFA group and 68.2% in the CBA group (Log-rank: p = 0.571) (Fig. 2).
Fig. 2.
Freedom from a composite outcome of a documented recurrence of any atrial tachyarrhythmia or a prescription of antiarrhythmic drugs in the RFA and CBA groups.
CBA cryoballoon ablation, RFA radiofrequency ablation.
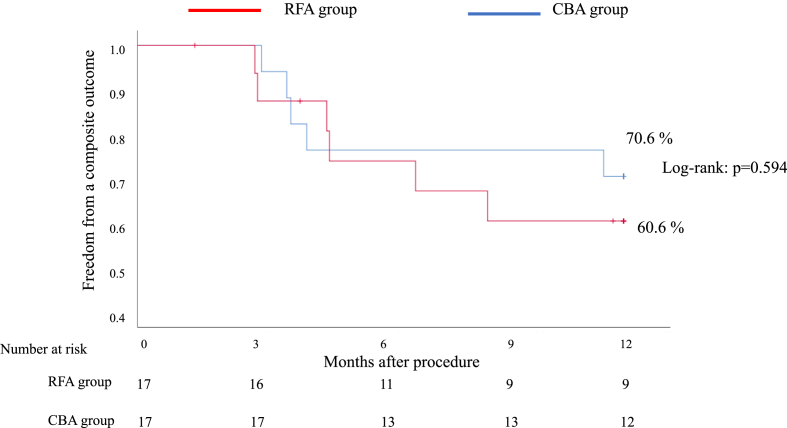
A subgroup analysis of PAF revealed that freedom from a composite outcome of a documented recurrence of any ATA or a prescription of an AAD at one year was 60.6% in the RFA group and 70.6% in the CBA group (Fig. 3).
Fig. 3.
Freedom from a composite outcome of a documented recurrence of any atrial tachyarrhythmia or a prescription of antiarrhythmic drugs in patients with paroxysmal atrial fibrillation.
CBA cryoballoon ablation, RFA radiofrequency ablation.
3.4. Complications
Two patients in RFA group and one patient in CBA group had procedure-related complications (Table 3). A puncture-site bleeding event which was necessary to extend the hospitalization occurred in one patient in RFA group. One patient in CBA group had pseudoaneurysm at a femoral artery. One patient in RFA group was required to be drained for pericardial effusion during CA.
Table 3.
Complications in RFA group and CBA group.
| RFA group(n = 21) | CBA group(n = 23) | p | |
|---|---|---|---|
| Complication, n(%) | 2(9.5) | 1(4.3) | 0.496 |
| Cardiac tamponade, n(%) | 1(4.8) | 0(0) | 0.290 |
| Groin site complication, n(%) | 1(4.8) | 1(4.3) | 0.947 |
RFA radiofrequency ablation, CBA cryoballoon ablation.
4. Discussion
4.1. Major finding
In this study, a composite outcome of a documented recurrence of any ATA or a prescription of an AAD in the CBA group was not statistically different from that in the RFA group. Although procedure-related complications were not different between the two groups, the procedure time was obviously shorter in the CBA group than in the RFA group.
4.2. AF in HD patients
Takigawa et al. reported the AF recurrence free rate at one year after the first RFA session for PAF in HD patients was 42.3%, and the one-year outcome after the final ablation was 64.7% [3]. In non-HD patients, it was 73.2% after the first session and 90.4% after the final ablation.3 Our results after the first ablation session for PAF were better than those reported by this previous study. One of the potential reasons could be that a non-contact force catheter was used in the aforementioned study. In the present research, we started using contact force catheters since 2016; this might be the reason for the AF recurrence in the previous study.
In our report, Arctic Front Advance or Arctic Front Advance PRO was used for CBA. The previously reported freedom from AF in the second-generation CBA (Arctic Front Advance) was better than that in the first-generation CBA (Arctic Front) at 15 months (90% in second generation vs 64% in the first generation) [14]. Recently, a report that published on using CBA for patients with HD found that the freedom from AF at 12 months was 79% [15]. Our result also supported that the freedom from AF in patients with HD after CBA might be better than RFA even though it was not statistically siginificant. Our report is the first to include PAF, PeAF, and LSAF. Our outcome was the composite endpoint of the recurrence of any ATA and the use of an AAD which was different from the previous report [15]
4.3. Touch-up ablation
In the CBA group, the rate of touch-up ablation was 20%, which was very high in our participant cohort. Based on our data, it is difficult to evaluate the relationship between the rate of touch-up ablation and HD. From a post-marketing surveillance report in Japan, 15.9% of patients needed treatment with focal ablation in addition to CBA [16]. From the North America registry study, 7.3% of patients needed treatment with focal ablation in addition to CBA [17]. Thus, there is a gap in the touch-up ablation rate in each report. One possible reason is the difference in institutional and operator volume. In our study, four out of five patients who needed a touch-up ablation underwent CA within one year since we started CBA in our institution. The learning curve of CBA in our hospital should be considered for this touch-up rate. Therefore, the high touch-up rate might be related with the institutional volume, not with HD.
4.4. Complications
The procedure-related complication rates from the FIRE and ICE study were 9.6% in the RFA group and 7.5% in the CBA group [4]. In an additional article that targeted HD patients receiving RFA, 6.1% of HD patients developed complications [3]. Our result revealed that the procedure-related complication rates was not statistically different between the two groups.
4.5. Reason that CBA might be better than RFA
The main point we should discuss in this article is the reason that the rate of AF recurrence was lower in the CBA group than in the RFA group. An optimized isolation might be based on the continuous lesion and the depth of the lesion. To our knowledge, no study has compared lesion depth between CBA and RFA. Only one case report performed a histological analysis on HD patients who underwent CBA [18]. Despite being a case report, we confirmed the presence of a transluminal wide lesion in the PV antrum after the second-generation CBA in HD patients. Kurose et al. reported that the lesion assessed by late gadolinium enhancement MRI after CBA was wider and more continuous than that after RFA [19]. When a large lesion such as in non-PVI is needed, RFA might be suitable. However, when the target lesion is on the PV, CBA might be suitable, especially for patients on dialysis because more continuous lesions could be confirmed.
Furthermore, in a previous report about AF recurrence, reconduction of PV was observed in 83.3% of HD patients with a recurrence of AF and in 86.4% of non-HD patients with a recurrence of AF. This result indicates that the durability of PVI is necessary in HD patients as well as in non-HD patients [20]
This report has several limitations. First, this study had a very small sample size. A large population should be assessed. Second, AF recurrence was not assessed using a loop recorder; thus, less AF cases might have been detected. Third, selection bias is possible. The choice of RFA or CBA was based on the anatomical characteristics found on CT scans. Patients who received RFA might have a more complex anatomy. Fourth, in the RFA group, several patients underwent CA using a received non-contact force catheter, which might have led to a higher AF recurrence. After CBA was accepted in Japan, our hospital started to frequently use CBA. The patients in the RFA group tended to be treated at an earlier time. Fifth, the use of AAD after CA was based on the physician’s discretion with or without a documented ATA. Physicians independently started AAD due to symptoms which suggested arrhythmias. Therefore, there is a high possibility that the patients who physicians treated with AAD after CA had a recurrence of ATA even though ATA was not documented. Furthermore, the discrepancy in the use of AAD in RFA group and CBA group in our could have affected the result of detecting ATA in each group. Finally, 95% patients in the RFA group received CTI, but this ablation was not performed because of an expected beneficial effect on preventing AF recurrence [21]
5. Conclusions
Our results suggest that patients on HD with AF who were treated with CBA tended to have better outcomes than patients treated with RFA. Therefore, CBA could be a suitable ablation method for HD patients. A large population study should be conducted to confirm our results.
Declarations
None.
Declaration of competing interest
None declared.
Acknowledgements
The authors are deeply grateful to the nursing staff, mechanical engineering staff, radiographers, and office administrators at Shonan Kamakura General Hospital.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Vazquez E., Sanchez-Perales C., Lozano C., Garcia-Cortes M.J., Borrego F., Guzman M. Comparison of prognostic value of atrial fibrillation versus sinus rhythm in patients on long-term hemodialysis. Am J Cardiol. 2003;92(7):868–871. doi: 10.1016/s0002-9149(03)00904-4. [DOI] [PubMed] [Google Scholar]
- 2.Genovesi S., Pogliani D., Faini A., Valsecchi M.G., Riva A., Stefani F. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46(5):897–902. doi: 10.1053/j.ajkd.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Takigawa M., Kuwahara T., Takahashi A., Kobori A., Takahashi Y., Okubo K. The impact of haemodialysis on the outcomes of catheter ablation in patients with paroxysmal atrial fibrillation. Europace. 2014;16(3):327–334. doi: 10.1093/europace/eut230. [DOI] [PubMed] [Google Scholar]
- 4.Kuck K.H., Brugada J., Furnkranz A., Metzner A., Ouyang F., Chun K.R. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 5.Lin W.S., Tai C.T., Hsieh M.H., Tsai C.F., Lin Y.K., Tsao H.M. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107(25):3176–3183. doi: 10.1161/01.Cir.0000074206.52056.2d. [DOI] [PubMed] [Google Scholar]
- 6.Lin W.S., Prakash V.S., Tai C.T., Hsieh M.H., Tsai C.F., Yu W.C. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation. 2000;101(11):1274–1281. doi: 10.1161/01.cir.101.11.1274. [DOI] [PubMed] [Google Scholar]
- 7.Yamada T., McElderry H.T., Epstein A.E., Plumb V.J., Kay G.N. One-puncture, double-transseptal catheterization manoeuvre in the catheter ablation of atrial fibrillation. Europace. 2007;9(7):487–489. doi: 10.1093/europace/eum070. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki S., Kuwahara T., Kobori A., Takahashi Y., Takei A., Sato A. Catheter ablation of atrial fibrillation in patients with valvular heart disease: long-term follow-up results. J Cardiovasc Electrophysiol. 2010;21(11):1193–1198. doi: 10.1111/j.1540-8167.2010.01812.x. [DOI] [PubMed] [Google Scholar]
- 9.Mattia L., Crosato M., Indiani S., Causin E., Licciardello C., Maria Squasi P.A. Prospective evaluation of lesion index-guided pulmonary vein isolation technique in patients with paroxysmal atrial fibrillation: 1-year follow-up. J Atr Fibrillation. 2018;10(6):1858. doi: 10.4022/jafib.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redfearn D.P., Trim G.M., Skanes A.C., Petrellis B., Krahn A.D., Yee R. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16(6):589–593. doi: 10.1111/j.1540-8167.2005.40825.x. [DOI] [PubMed] [Google Scholar]
- 11.Rottner L., Fink T., Heeger C.H., Schluter M., Goldmann B., Lemes C. Is less more? Impact of different ablation protocols on periprocedural complications in second-generation cryoballoon based pulmonary vein isolation. Europace. 2018;20(9):1459–1467. doi: 10.1093/europace/eux219. [DOI] [PubMed] [Google Scholar]
- 12.John R.M., Kapur S., Ellenbogen K.A., Koneru J.N. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm. 2017;14(2):184–189. doi: 10.1016/j.hrthm.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Franceschi F., Dubuc M., Guerra P.G., Delisle S., Romeo P., Landry E. Diaphragmatic electromyography during cryoballoon ablation: a novel concept in the prevention of phrenic nerve palsy. Heart Rhythm. 2011;8(6):885–891. doi: 10.1016/j.hrthm.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Greiss H., Berkowitsch A., Wojcik M., Zaltsberg S., Pajitnev D., Deubner N. The impact of left atrial surface area and the second generation cryoballoon on clinical outcome of atrial fibrillation cryoablation. Pacing Clin Electrophysiol. 2015;38(7):815–824. doi: 10.1111/pace.12637. [DOI] [PubMed] [Google Scholar]
- 15.Takamiya T., Nitta J., Inaba O., Sato A., Inamura Y., Kato N. Cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation in hemodialysis patients. Heart Ves. 2020 doi: 10.1007/s00380-020-01646-5. [DOI] [PubMed] [Google Scholar]
- 16.Okumura K., Matsumoto K., Kobayashi Y., Nogami A., Hokanson R.B., Kueffer F. Safety and efficacy of cryoballoon ablation for paroxysmal atrial fibrillation in Japan- results from the Japanese prospective post-market surveillance study. Circ J. 2016;80(8):1744–1749. doi: 10.1253/circj.CJ-16-0285. [DOI] [PubMed] [Google Scholar]
- 17.Knight B.P., Novak P.G., Sangrigoli R., Champagne J., Dubuc M., Adler S.W. Long-Term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: final results from STOP AF post-approval study. JACC Clin Electrophysiol. 2019;5(3):306–314. doi: 10.1016/j.jacep.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Hirao T., Nitta J., Adachi A., Takahashi Y., Goya M., Hirao K. First confirmation of histologic changes in the human heart after cryoballoon ablation. HeartRhythm Case Rep. 2019;5(2):93–96. doi: 10.1016/j.hrcr.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurose J., Kiuchi K., Fukuzawa K., Mori S., Ichibori H., Konishi H. The lesion characteristics assessed by LGE-MRI after the cryoballoon ablation and conventional radiofrequency ablation. J Arrhythm. 2018;34(2):158–166. doi: 10.1002/joa3.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi M., Kaneko S., Shimano M., Ohashi T., Kubota R., Takeshita K. Efficacy and safety of radiofrequency catheter ablation for atrial fibrillation in chronic hemodialysis patients. Nephrol Dial Transplant. 2014;29(1):160–167. doi: 10.1093/ndt/gft233. [DOI] [PubMed] [Google Scholar]
- 21.Shah D.C., Sunthorn H., Burri H., Gentil-Baron P. Evaluation of an individualized strategy of cavotricuspid isthmus ablation as an adjunct to atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2007;18(9):926–930. doi: 10.1111/j.1540-8167.2007.00896.x. [DOI] [PubMed] [Google Scholar]