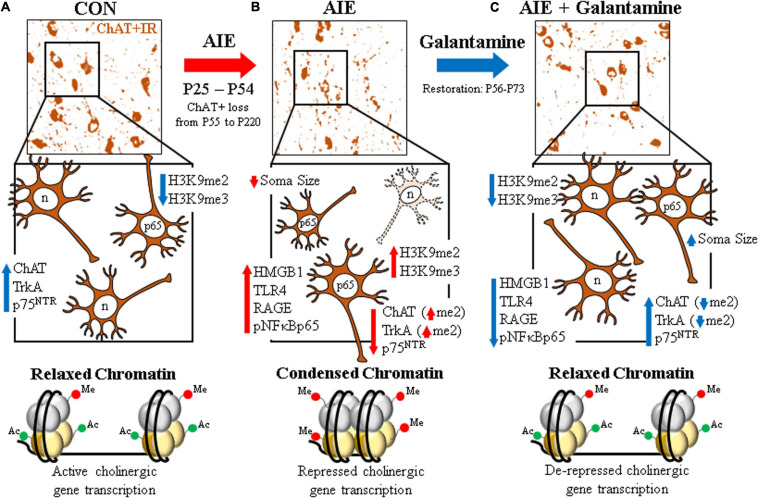
FIGURE 8.
Schematic depicting the proposed mechanism underlying the persistent adolescent intermittent ethanol (AIE)-induced loss of basal forebrain cholinergic neurons. (A) In the naïve basal forebrain, cholinergic neurons express choline acetyltransferase (ChAT), the high-affinity nerve growth factor (NGF) receptor tropomyosin receptor kinase A (TrkA), and the low-affinity NGF receptor p75 neurotrophin receptor (p75NTR) (Vetreno et al., 2019). (Top) ChAT + IR neurons in the CON-treated adult (P73) basal forebrain. (Middle) Schematic depicting ChAT + IR basal forebrain cholinergic neurons in orange. Note that “n” in the nucleus represents neuronal NeuN immunoreactivity. Constitutive expression of proinflammatory signaling molecules and histone methylation markers are relatively low in the naïve basal forebrain. (Bottom) Schematic depicting relaxed, open chromatin allowing active transcription of cholinergic genes and maintenance of the cholinergic phenotype. (B) AIE causes the loss of ChAT + IR neurons in the adolescent (P55) basal forebrain that persists into adulthood (P200) (Vetreno et al., 2014). (Top) ChAT + IR neuron loss and shrinkage of the residual cholinergic neurons in the adult (P73) AIE-treated basal forebrain. (Middle) AIE-induced loss of ChAT-, TrkA-, and p75NTR-immunoreactive basal forebrain neurons (dashed neuron lacking ChAT [orange]) as well as somal shrinking of the residual ChAT + IR cholinergic neurons. AIE increased expression of the endogenous ligand HMGB1, the HMGB1 receptors TLR4 and RAGE, and phosphorylation of the proinflammatory transcription factor NF-κB p65 in the adult basal forebrain, and neuroimmune signaling might alter gene expression in part through epigenetic mechanisms (Montesinos et al., 2016). Note that “n” in the nucleus represents NeuN + IR, which was unchanged by AIE, consistent with the loss of the cholinergic phenotype and not cell death (Vetreno et al., 2019). AIE increased expression of histone 3 methylation markers (i.e., H3K9me2 and H3K9me3) and dimethylation of lysine 9 of histone 3 (H3K9me2) associated with promoter regions on the ChAT and TrkA genes in the adult basal forebrain. (Bottom) Schematic depicting condensed, closed chromatin repressing transcription of cholinergic genes. (C) Galantamine treatment from P57 to P72 restored the AIE-induced loss of basal forebrain cholinergic neurons. (Top) Galantamine restoration of the AIE-induced loss of ChAT + IR neurons and somal shrinkage of the residual cholinergic neurons in the adult (P73) basal forebrain. (Middle) Restoration of ChAT-, TrkA, and p75NTR-immunoreactive basal forebrain neurons, and reversal of cholinergic neuron shrinkage in the adult basal forebrain of AIE-treated subjects. Restorative galantamine treatment reversed the AIE-induced increased expression of proinflammatory neuroimmune signaling molecules (i.e., HMGB1, RAGE/TLR4, and pNF-κB p65), histone methylation markers (i.e., H3K9me2 and H3K9me3), and increase of H3K9me2 in promoter regions of both the ChAT and TrkA genes in the adult basal forebrain. These data suggest that galantamine is a potential treatment modality for maladaptive changes in neural architecture in the adult basal forebrain that may recover cognitive deficits associated with cholinergic system dysfunction. (Bottom) Schematic depicting galantamine restoration of condensed chromatin to relaxed, open chromatin state allowing de-repression and active transcription of cholinergic genes and restoration of the cholinergic neuron phenotype.