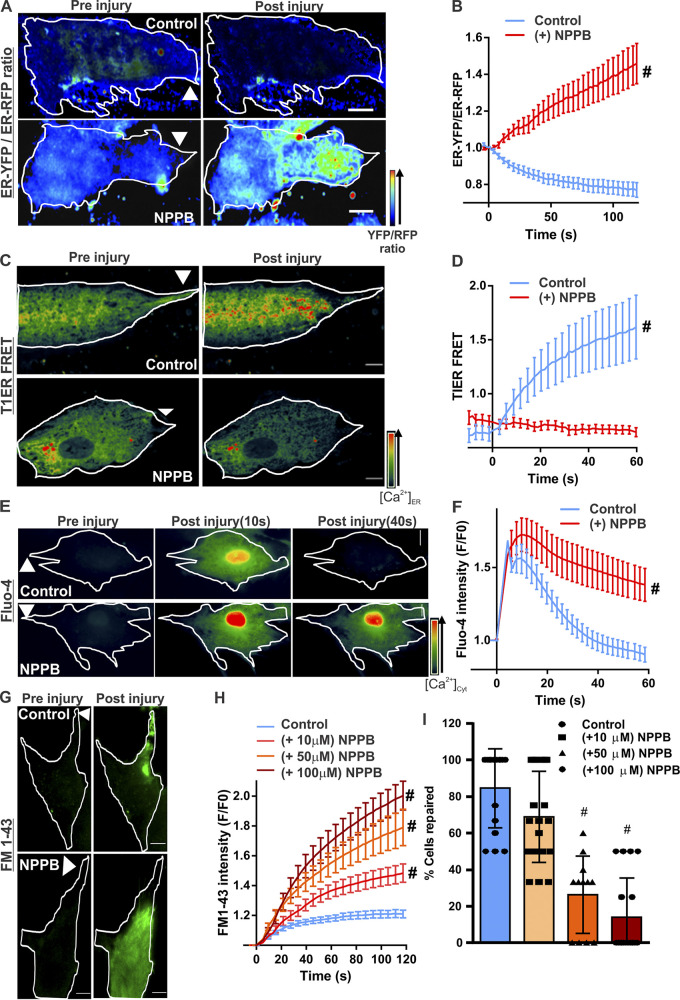
Figure 2.
Ca2+ homeostasis during PMR requires CaCC activity. Human myoblasts expressing KDEL-YFP and KDEL-RFP were treated with vehicle (control) or a CaCC blocker (50 µM NPPB). Quenching of YFP fluorescence by Cl− entry into the ER was monitored by ratiometric measurement of Cl−-sensitive YFP and Cl−-insensitive RFP fluorescence. (A) Pseudocolored images of cells before or 60s after laser injury (injury site marked by arrowheads). (B) Quantification of the normalized YFP/RFP ratio in control and NPPB-treated myoblasts. n = 18 (control) and 16 (NPPB) cells; #, P < 0.0001. (C and D) Pseudocolored images (C) and quantification (D) of T1ER FRET ratios before and after injury of myoblasts treated with vehicle (control) or NPPB (50 µM). Arrowheads indicate site of injury. n = 20 (control) and 17 (NPPB 50 µM) cells; #, P < 0.0001. (E and F) Pseudocolored images (E) and plots (F) showing change in [Ca2+]c monitored by Fluo-4 labeling of human myoblasts treated with vehicle (control) or NPPB. n = 23 (control) and 24 (NPPB) cells; #, P < 0.0001. (G and H) Images (G) and plots (H) showing FM1–43 dye entry into healthy myoblasts treated with vehicle (control) or NPPB (at indicated doses) and focally injured by laser (site marked with arrowheads). (I) Percentage of myoblasts that repaired from PM injury after treatment with indicated NPPB doses. n = 16 (control), 19 (NPPB 10 µM), 12 (NPPB 50 µM), and 18 (NPPB 100 µM) cells; #, P < 0.0001. Error bars represent SD for the plot in I and SEM for all other plots. Scale bars, 10 µm.