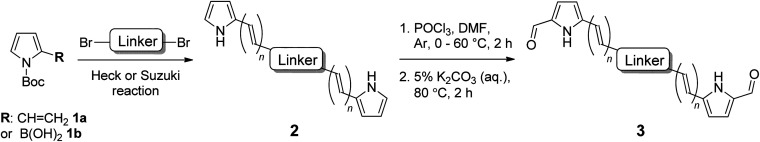
Table 1. Synthesis of a novel series of bis(pyrrolic) ligands (3a–3k).
| ||||||
| Entry | Pyrrole | Linker |
n | Yield of 2 b (%) | Yield of 3 b (%) | |
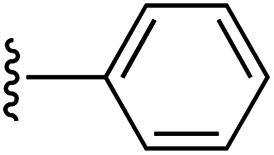
| 1 | 1a | a a |
|
1 | 64 (2a) a , c | 86 (3a) a , g |
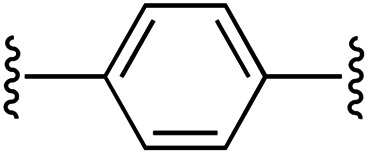
| 2 | 1a | b |
|
1 | 86 (2b) d | 85 (3b) h |
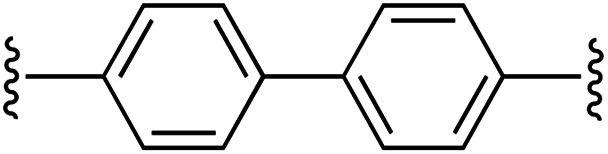
| 3 | 1a | c |
|
1 | 94 (2c) e | 85 (3c) h |
| 4 | 1a | d |
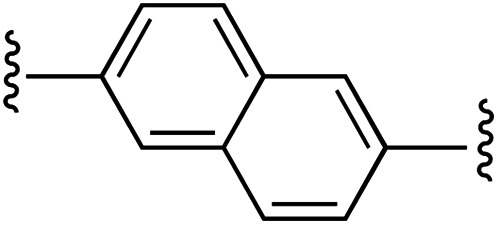
|
1 | 97 (2d) d | 76 (3d) h |
| 5 | 1a | e |
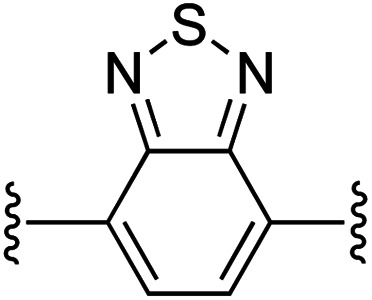
|
1 | 91 (2e) d | 97 (3e) h |
| 6 | 1a | f |
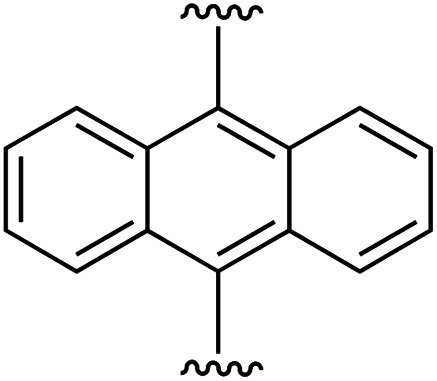
|
1 | 97 (2f) d | 87 (3f) h |
| 7 | 1a | g |
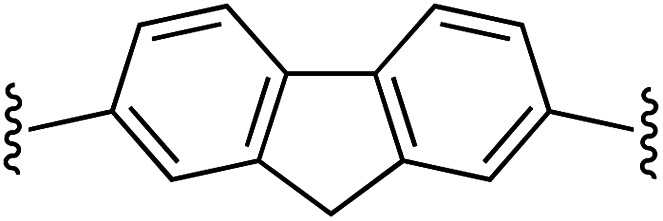
|
1 | 100 (2g) d | 94 (3g) h |
| 8 | 1a | h |
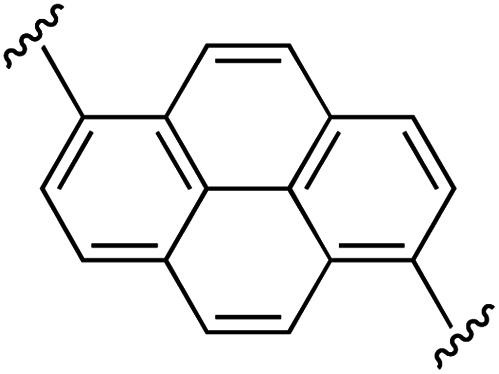
|
1 | 100 (2h) d | 95 (3h) h |
| 9 | 1a | i |
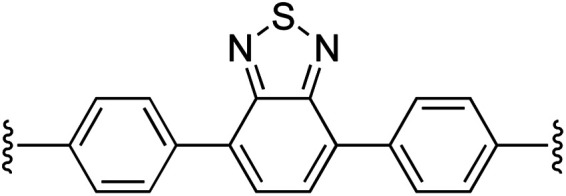
|
1 | 100 (2i) d | 92 (3i) h |
| 10 | 1b | j |
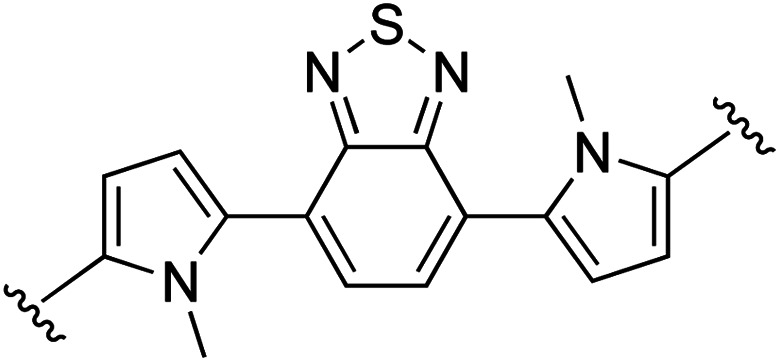
|
0 | 85 (2j) e | 84 (3j) h |
| 11 | 1b | k |
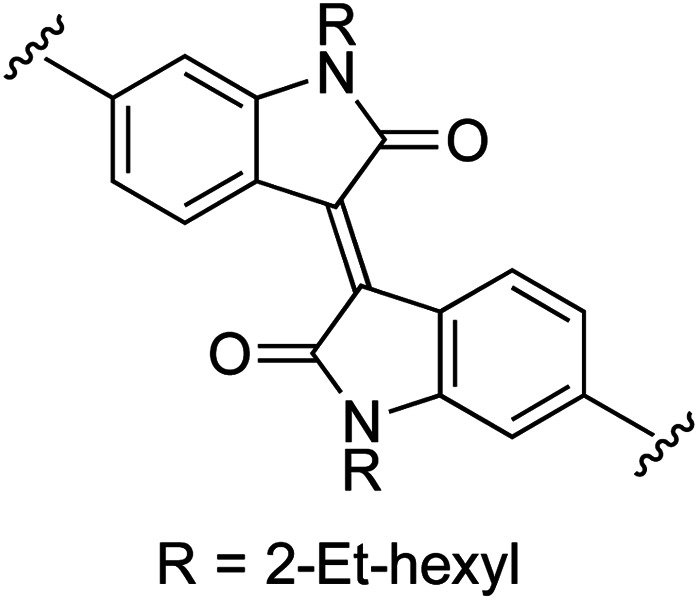
|
0 | 53 (2k) e , f | 90 (3k) h |
aCompounds 2a (see Scheme 1) and 3a are mono-pyrroles (pyrrole-CH = CHPh).
bIsolated yield.
cHeck reaction conditions: 1 equiv. 1a, Pd(OAc)2, 2,4-pentanedione, K2CO3, DMF, Ar, 130 °C, 3 h.
dHeck reaction, 2 equiv. 1a, 6 h.
eSuzuki reaction conditions: Pd(PPh3)4, K2CO3, DMF, 110 °C, 24 h.
fSuzuki reaction, 115 °C for 18 h then 125 °C for 5 h.
gVilsmeier reaction, 1 equiv. POCl3.
hVilsmeier reaction, 2 equiv. POCl3.
