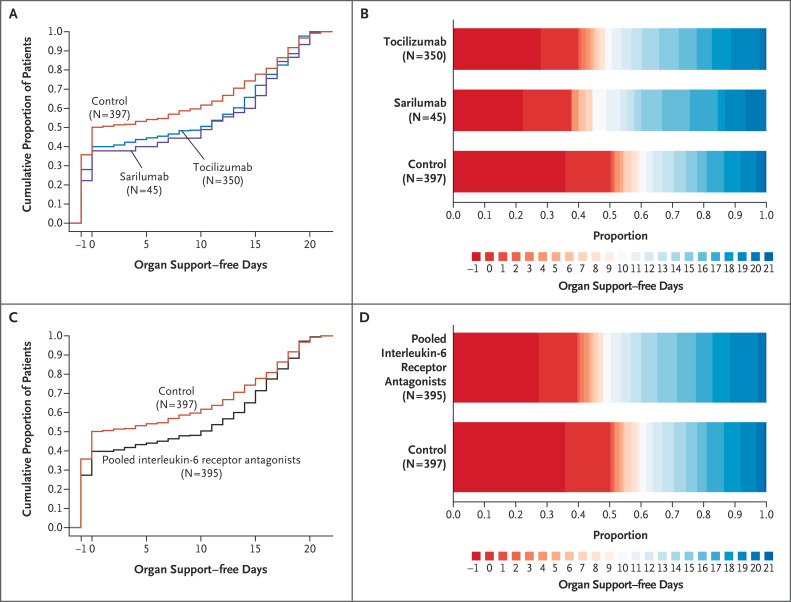
Figure 2. Distributions of Organ Support–free Days.
Panel A shows the cumulative proportion of patients for each intervention group according to day, with death shown first. Curves that rise more gradually indicate a more favorable distribution in the number of days alive and free of organ support. The height of each curve at “−1” indicates the in-hospital mortality for each intervention. The height of each curve at any time point indicates the proportion of patients who had that number of organ support–free days or fewer (e.g., the height at day 10 indicates the proportion of patients with ≤10 organ support–free days). The difference in the height of the curves at any point represents the difference in the percentile in the distribution of organ support–free days associated with that number of days alive and free of organ support. Panel B shows organ support–free days as horizontally stacked proportions according to intervention group. Red represents worse outcomes, and blue represents better outcomes. The median adjusted odds ratios from the primary analysis, which used a Bayesian cumulative logistic model, were 1.64 (95% credible interval, 1.25 to 2.14) and 1.76 (95% credible interval, 1.17 to 2.91) for the tocilizumab and sarilumab groups, respectively, as compared with control, yielding probabilities of superiority to control of more than 99.9% and of 99.5%, respectively. Panels C and D are similar to Panels A and B but with the tocilizumab and sarilumab groups pooled together. The median adjusted odds ratio was 1.65 (95% credible interval, 1.27 to 2.14), yielding a probability of superiority to control of more than 99.9%.