Abstract
We followed 35 children meeting a research definition for unconfirmed tuberculosis (TB) but in whom a pediatric pulmonologist did not diagnose or treat TB. After a median follow-up of 16.4 months, most children were not diagnosed with TB following a comprehensive evaluation. However, 2 were diagnosed with TB, demonstrating high TB risk (6%; exact 95% CI, 1%–19%). In some contexts, researchers may wish to supplement these research definitions with clinical decision data and longitudinal follow-up in order to improve specificity.
Keywords: case definition, diagnosis, pediatric tuberculosis
An estimated 400 children die each day of TB [1]. Timely diagnosis and treatment are critical to preventing mortality in this group [2, 3]. However, challenges remain in diagnosing pediatric TB [4]. Young children are often unable to produce sputum samples, and microbiological assays have low sensitivity in children even when sputum is available [5]. This study aimed to trace a cohort of children who met a standardized research case definition for unconfirmed TB, but in whom the disease was not diagnosed following a comprehensive evaluation. We quantified the frequency of subsequent TB diagnoses in this group, either due to existing undiagnosed TB (ie, a missed diagnosis) or incident TB.
METHODS
Context
This work was nested within a pediatric TB diagnostic study in Lima, Peru [6–9]. From May 2015 through February 2018, the study enrolled children <15 years of age who presented either passively, or following household contact tracing, to a Ministry of Health (MoH) primary-level facility. Eligible children had both a close adult contact with TB in the previous 2 years and at least 1 TB symptom [10]. Children underwent routine TB diagnostic procedures per Peruvian MoH guidelines, which include acid-fast smear and mycobacterial culture of at least 2 respiratory specimens (sputum or gastric aspirate), tuberculin skin testing (TST), and chest radiography. A single MoH pediatric pulmonologist reviewed chest radiographs (frontal and lateral) for each child using a standardized tool, noted the type and location of any abnormalities, and made a determination of “normal,” “abnormal, consistent with TB,” or “abnormal, not consistent with TB.” The same pediatric pulmonologist evaluated each case with respect to these results, exam findings, and clinical and epidemiologic history and decided whether to treat for TB. Peruvian National TB Program (NTP) guidelines define a case of probable (ie, bacteriologically unconfirmed) TB as one in which at least 3 of the following criteria are met: (1) presence of TB symptoms, (2) exposure to an infectious active TB case, (3) positive TST, (4) chest radiography consistent with TB, and (5) evidence from other supporting exams (ie, for extrapulmonary TB) [11]. Among children in whom active TB disease is ruled out, isoniazid preventive treatment is indicated for those younger than 5 years old with a pulmonary TB contact and for those 5 to 19 years old who have both a pulmonary TB contact and a TST result ≥10 mm [11].
Participants
In this substudy, we enrolled children who met the National Institutes of Health (NIH) research case definition for microbiologically unconfirmed pediatric TB [12], but in whom TB was not diagnosed or treated by the pediatric pulmonary specialist overseeing the case, and conducted a re-evaluation for TB disease. NIH case definitions for intrathoracic TB define a case of unconfirmed pediatric TB as one in which bacteriologic confirmation was not obtained and that meets 2 of the following criteria: symptoms or signs suggestive of TB, abnormal chest radiograph consistent with TB, close contact with a TB case or immunologic evidence of infection with Mycobacterium tuberculosis, or a positive response to TB treatment. Because TB symptoms and close contact with an adult with TB were required for enrollment in the larger diagnostic study, all children technically met the definition for unconfirmed TB. For the purpose of this substudy, we required that children meet a third NIH criterion, specifically that their chest radiograph was consistent with TB. This approach aligns with Peruvian NTP pediatric TB case definitions, which require that cases meet at least 3 criteria [11].
Data Collection
From September 2017 to September 2018, following informed consent, we conducted interviews with children and caregivers to determine whether children had experienced persistent TB symptoms in the previous 30 days. Children with symptoms received a chest radiograph. If a pediatric pulmonologist suspected TB based on the radiograph, a sample of sputum (or gastric aspirate if sputum could not be produced spontaneously) was collected for acid-fast smear and mycobacterial culture using BACTEC MGIT 960 (Becton-Dickinson, Franklin Lakes, NJ, USA). Following these procedures and a clinical evaluation, an MoH pediatric pulmonologist, who was not the physician who conducted the initial clinical evaluation, made a diagnostic determination. The pediatric pulmonologist had access to the initial chest radiograph for comparison purposes.
Patient Consent Statement
Written informed consent was obtained from a caregiver; written assent was obtained from children ≥8 years old. The design of the study was approved by the Ethics Committee of the National Health Institute of Peru (Lima, Peru) and the Human Research Ethics Committee of Harvard Medical School (Boston, MA, USA).
RESULTS
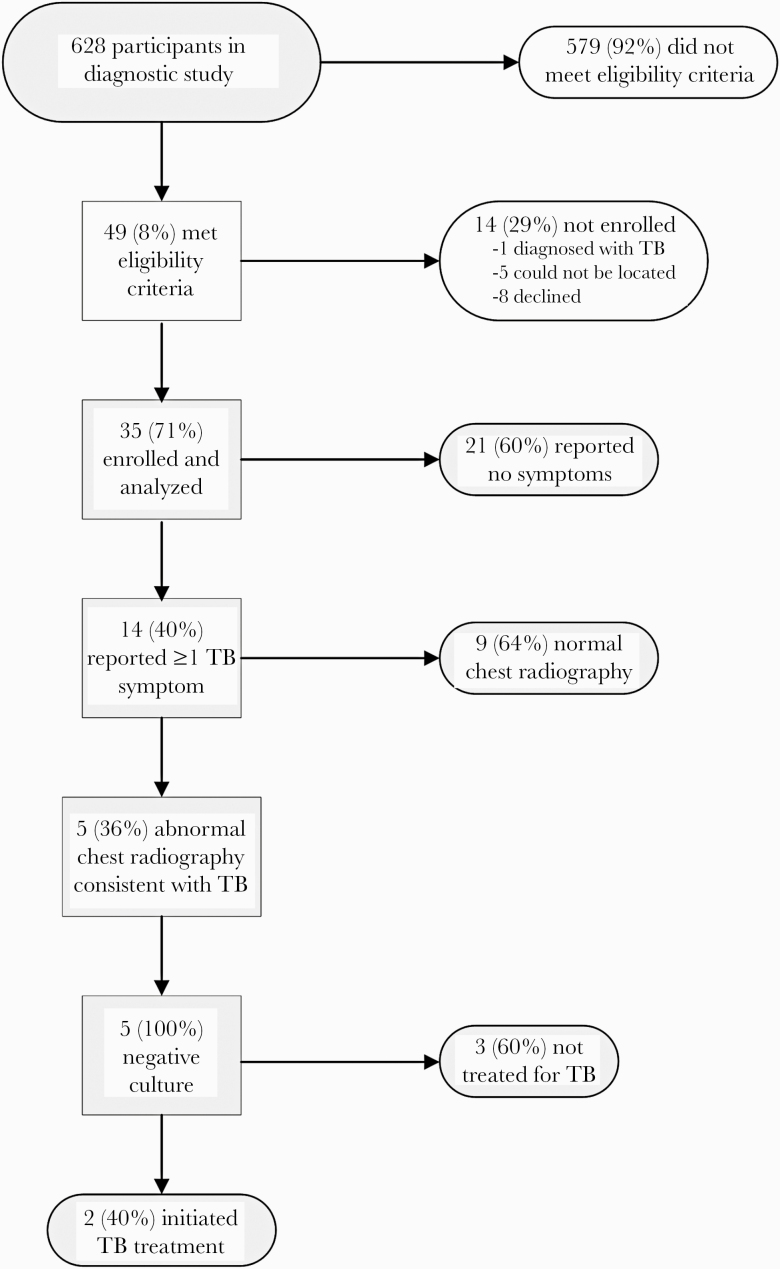
Forty-nine children met eligibility criteria (Figure 1). Among them, 5 (10%) could not be located. Of 44 invited to participate, 35 (80%) enrolled. The remaining 9 did not participate due to having moved outside the country (n = 1), time constraints (n = 6), refusal (n = 1), and a recent TB diagnosis (n = 1). The median age (range, interquartile range [IQR]) of the 35 participants was 3.6 (0.8–12.7, 1.8–9.1) years, and there was a median (IQR) of 16.4 (12.2–21.8) months between the initial and follow-up evaluations. Table 1 describes characteristics of those who did and did not participate. The frequency of isoniazid preventive therapy was low in both groups (17% and 8%, respectively). Participants were generally similar to those diagnosed with unconfirmed TB by a study clinician, with the exception that TST positivity tended to be higher among those with an unconfirmed diagnosis (67%, vs 47% among participants).
Figure 1.
Flowchart of study enrollment and follow-up. Abbreviation: TB, tuberculosis.
Table 1.
Descriptive characteristics of participants, non-participants, and children in whom unconfirmed tuberculosis was diagnosed
| Characteristic | Participants (N=35) | Non-participants (eligible but not enrolled, N=13)a | Unconfirmed TB (N=103) |
|---|---|---|---|
| Age in years, median (IQR) | 3.6 (1.8–9.1) | 4.0 (1.9–5.2) | 4.7 (2.3–8.5) |
| Age group, n (%) | |||
| 0–4 years | 20 (57) | 9 (69) | 52 (50) |
| 5–10 years | 11 (31) | 3 (23) | 42 (41) |
| 11–14 years | 4 (11) | 1 (8) | 9 (9) |
| Female, n (%) | 15 (43) | 1 (8) | 55 (53) |
| Symptoms at initial evaluation, n (%) | |||
| fever | 2 (6) | 1 (8) | 13 (13)b |
| weight loss | 5 (14) | 1 (8) | 19 (19)b |
| night sweats | 2 (6)c | 1 (8) | 5 (5)b |
| cough | 22 (63) | 5 (38) | 63 (63)b |
| Contact was child’s mother, n (%) | 13 (37) | 6 (46) | 32 (33)d |
| BMI, median (IQR) | 16.5 (15.7–18.4) | 17.5 (17.0–18.6) | 16.6 (15.4–18.6) |
| Socioeconomic score, median (IQR)e | −0.40 (-0.81–0.42) | 0.44 (-1.57–1.38) | −0.16 (-1.60–1.05) |
| Positive TST result, n (%)f,g | 16 (47) | 5 (42) | 66 (67) |
| Chest radiography consistent with TB | 35 (100)h | 13 (100)h | 88 (88)b |
| BCG (self-report), n (%)i | 26 (96) | 12 (100) | 93 (98) |
| HIV, n (%) | 0 (0) | 0 (0) | 0 (0)b |
| Received isoniazid preventive treatment | 6 (17) | 1 (8) | Not applicable |
Abbreviations: BCG, Bacillus Calmette-Guérin; BMI, body mass index; IQR, interquartile range; TB, tuberculosis; TST, tuberculin skin testing.
aWe excluded from this column the child who did not participate because he was diagnosed with TB.
bN=100.
cN=34.
dN=97.
eBased on principal component analyses [18].
fN=34, N=12, N=98 for children who did and did not enroll and who had unconfirmed TB, respectively.
gSkin reactions with an induration of 10 mm or greater were considered positive.
hRequired for participation.
iN=27, N=12, and N=95 for children who did and did not enroll and who had unconfirmed TB, respectively.
At the time of re-evaluation, 14 children (40%) had at least 1 symptom of TB and underwent chest radiograph. Of these, 5 (36%) had abnormal chest radiograph findings consistent with TB, including interstitial changes (n = 2), nodular infiltrates (n = 2), calcification (n = 2), and perihilar lymphadenopathy (n = 3). All 5 children had negative smear and culture results. Two of these 5 children, 6% of all those re-evaluated (exact 95% CI, 1%–19%), were diagnosed with TB based on their re-evaluation, one 10 and the other 30 months post–initial evaluation. Both children—a 10-year-old male and an 11-year-old female—had productive cough and unexplained fatigue that had resolved after the initial evaluation but then returned. On chest radiograph, 1 had nodular infiltrates and perihilar lymphadenopathy, and the other had calcification and perihilar lymphadenopathy. A pulmonologist from the primary-level health facility discontinued TB treatment in 1 of the 2 children after 1 week and initiated antibiotics for “bronchitis.”
DISCUSSION
Our study illustrates a number of practical challenges to diagnosing and researching pediatric TB. The first relates to the application of NIH case definitions. We identified children with TB symptoms, a household contact, and chest radiograph consistent with TB (ie, a case of unconfirmed TB according to NIH case definitions) who were not diagnosed with TB by a pediatric pulmonary specialist. A median of 16.4 months after initial evaluation, TB was ruled out for a second time in the majority of children who were located and screened. This raises the possibility that these case definitions may overcall TB in some settings, which has important implications for the studies using them. When appropriate, investigators may wish to improve specificity by combining these case definitions with data from a clinical evaluation or prescription of treatment. One potential contributor to the observed discrepancies between NIH case definitions and clinical decision-making is that the NIH case definitions were not designed for the setting of active case finding [13, 14], and some of children presented to health facilities following contact tracing activities. Another consideration is that NIH case definitions apply equal weight to each criterion, while clinicians may implicitly or explicitly weigh factors differently or consider factors beyond those noted in case definitions (eg, access to care). Future work could build on these research case definitions to expand their use in different contexts (eg, active case finding). Weighting of criteria and differentiation between an absence of data vs the explicit absence of a finding are just 2 potential pathways that could be explored to optimize performance in different settings.
A second important finding is that while most children did not have TB on follow-up, 2 (6%) were diagnosed with TB as a result of the study’s active follow-up, suggesting either missed diagnoses or incident TB. Our small sample size resulted in wide 95% CIs, indicating that this frequency may be anywhere from 1% to 19%. If we count the third child who was eligible for enrollment but not enrolled because he was diagnosed with TB after the initial study evaluation but before our active follow-up, the frequency of TB in this group was 8% (3/36; 95% CI, 2%–22%). Thus, children who meet the NIH case definition for unconfirmed TB are at high risk for TB and should be closely monitored even if TB is initially ruled out. Of note, a sizable proportion (27% (13/49)) of children eligible for inclusion could not be located or their caregivers declined to participate. On the whole, participants and nonparticipants were similar; however, if nonparticipants had a risk of TB that was higher or lower than those who were enrolled, results would be biased.
Last, we highlight the child diagnosed at follow-up, but in whom TB treatment was suspended after 1 week by a different pulmonologist. This scenario exemplifies the uncertainty of diagnosing TB in the absence of an accurate test, even among experienced specialists. Inter-rater reliability of chest radiography readings is suboptimal [15], and hesitation and conflicting diagnostic opinions among health providers may lead to delays in prescribing effective treatment [16], including IPT. Appropriately, providers may hold off on IPT if there is uncertainty about whether a child has TB. In our cohort, this was evidenced by a lower frequency of IPT among these children relative to a programmatic cohort in Lima, Peru, from the same time period [17].
A limitation of this study is that were unable to determine whether new TB diagnoses were initially missed or represent incident TB. A second limitation is the lack of additional follow-up among children enrolled in this substudy who were not treated for TB, including the 1 in whom TB treatment was discontinued. Data were collected as part of a research study and within a well-functioning NTP; children had access to pulmonologists and a broad range of diagnostic tools, including liquid culture, gastric aspiration, chest radiography, and tuberculin skin testing. Therefore, these results may represent a “best case” scenario in which a relatively small percentage of TB cases would be expected to have been missed. In the absence of an accurate diagnostic tool for TB, regular longitudinal monitoring of children at high risk will be essential to prompt diagnosis and treatment.
Acknowledgments
We acknowledge and thank the children, caregivers, health providers, and Socios En Salud staff members who participated in the study. We thank Zibiao Zhang for programming support.
Financial support. This work was supported by the American Lung Association (grant SB-412572) and the National Institutes of Health’s Center of Excellence in Translational Research (grant U19 AI109755).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1. Accessed 10 June 2019. [Google Scholar]
- 2. Cruz AT, Starke JR. Pediatric tuberculosis. Pediatr Rev 2010; 31:13–25; quiz 25–6. [DOI] [PubMed] [Google Scholar]
- 3. Mandal N, Anand PK, Gautam S, et al. Diagnosis and treatment of paediatric tuberculosis: an insight review. Crit Rev Microbiol 2017; 43:466–80. [DOI] [PubMed] [Google Scholar]
- 4. Graham SM. Research into tuberculosis diagnosis in children. Lancet Infect Dis 2010; 10:581–2. [DOI] [PubMed] [Google Scholar]
- 5. Ioos V, Cordel H, Bonnet M. Alternative sputum collection methods for diagnosis of childhood intrathoracic tuberculosis: a systematic literature review. Arch Dis Child 2019; 104:629–35. [DOI] [PubMed] [Google Scholar]
- 6. Tafur KT, Coit J, Leon SR, et al. Feasibility of the string test for tuberculosis diagnosis in children between 4 and 14 years old. BMC Infect Dis 2018; 18:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mesman AW, Soto M, Coit J, et al. Detection of Mycobacterium tuberculosis in pediatric stool samples using TruTip technology. BMC Infect Dis 2019; 19:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flores JA, Calderón R, Mesman AW, et al. Detection of Mycobacterium tuberculosis DNA in buccal swab samples from children in Lima, Peru. Pediatr Infect Dis J 2020; 39:e376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coit J, Mendoza M, Pinedo C, et al. Performance of a household tuberculosis exposure survey among children in a Latin American setting. Int J Tuberc Lung Dis 2019; 23:1223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cuevas LE, Browning R, Bossuyt P, et al. Evaluation of tuberculosis diagnostics in children: 2. Methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children. Consensus from an expert panel. J Infect Dis 2012; 205(Suppl 2):S209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dirección General de Salud de las Personas. Estrategia Sanitaria Nacional de Prevención y Control de la Tuberculosis. Norma técnica de salud para la atención integral de las personas afectadas por tuberculosis. Available at: http://www.minsa.gob.pe. Accessed 11 January 2021.
- 12. Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015; 61( Suppl 3):S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiseman CA, Mandalakas AM, Kirchner HL, et al. Novel application of NIH case definitions in a paediatric tuberculosis contact investigation study. Int J Tuberc Lung Dis 2015; 19:446–53. [DOI] [PubMed] [Google Scholar]
- 14. Beneri CA, Aaron L, Kim S, et al. ; P1041 team . Understanding NIH clinical case definitions for pediatric intrathoracic TB by applying them to a clinical trial. Int J Tuberc Lung Dis 2016; 20:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andronikou S, Grier D, Minhas K. Reliability of chest radiograph interpretation for pulmonary tuberculosis in the screening of childhood TB contacts and migrant children in the UK. Clin Radiol 2021; 76:122–8. [DOI] [PubMed] [Google Scholar]
- 16. Coit J, Wong M, Galea JT, et al. Uncovering reasons for treatment initiation delays among children with TB in Lima, Peru. Int J Tuberc Lung Dis 2020; 24:1254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otero L, Battaglioli T, Ríos J, et al. Contact evaluation and isoniazid preventive therapy among close and household contacts of tuberculosis patients in Lima, Peru: an analysis of routine data. Trop Med Int Health 2020; 25:346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flores JA, Coit J, Mendoza M, et al. Is exclusive breastfeeding for six-months protective against pediatric tuberculosis? Glob Health Action 2021; 14:1861922. [DOI] [PMC free article] [PubMed] [Google Scholar]