Abstract
Purpose
The prevalence of nonalcoholic fatty liver disease (NAFLD), which has recently become known as metabolic-associated fatty liver disease (MAFLD), has risen. However, pharmacotherapies for this disease have not been approved. Electromagnetic fields (EMFs) have excellent bioeffects on multiple diseases. However, the effects of EMFs on NAFLD are unknown. This study investigated the bioeffects of EMF exposure on insulin resistance, liver redox homeostasis and hepatic steatosis in db/db mice.
Methods
Animals were sacrificed after EMF exposure for 8 weeks. The fasting blood glucose and insulin levels in the serum were tested. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by a formula. The levels of MDA, GSSG and GSH, biomarkers of redox, were assessed. The activities of CAT, SOD and GSH-Px were assessed. The body and liver weights were measured. Hepatic lipid accumulation was observed by Oil Red O staining. Hepatic CAT, GR, GSH-Px, SOD1, SOD2 and SREBP-1 expression was determined by Western blotting.
Results
EMF exposure ameliorated insulin resistance and oxidative stress in the liver by downregulating the MDA and GSSG levels, increasing the reduced GSH levels, and promoting the GSH-Px levels in db/db mice. In addition, liver weight and triglyceride (TG) levels were reduced by EMF exposure. Simultaneously, EMF exposure improved hepatic steatosis by downregulating the protein expression of SREBP-1c.
Conclusion
The present findings suggest that EMF exposure has positive effects in the treatment of NAFLD.
Keywords: nonalcoholic fatty liver disease, electromagnetic fields, hepatic steatosis, oxidative stress, insulin resistance
Introduction
The prevalence of Nonalcoholic fatty liver disease (NAFLD), which has recently become known as metabolic-associated fatty liver disease (MAFLD), has risen, but its pathophysiology remains unknown.1,2 To date, the response rates of several phase 2B and phase 3 clinical trials for treating NAFLD appear modest.2 Thus, no pharmacotherapies have been approved by the Food and Drug Administration (FDA).3,4 Aberrant redox signalling affects the pathophysiology of NAFLD, which is becoming increasingly understood.5,6 Oxidative stress can reduce insulin sensitivity and increase hepatic triglyceride (TG) content.7,8 Hepatic insulin resistance can result in hepatic steatosis by causing lipid metabolism disorders, which further lead to systemic IR.9 Excessive hepatic lipid accumulation further exacerbates IR.5 Thus, re-establishing systemic redox homeostasis plays an important role in treating NAFLD.8 Unfortunately, intervention in redox systems remains a clinical challenge because of side effects that negatively impact adherence to treatment.10 Re-establishing redox homeostasis requires new methods.
Recent literature has reported that static magnetic fields combined with electric fields can promote insulin sensitivity by modulating redox homeostasis for the treatment of T2DM.11 Electromagnetic fields (EMFs) include both electric and magnetic fields, so we reasoned that EMFs may improve IR and oxidative stress in NAFLD. However, evidence supporting the bioeffect of EMFs in insulin sensitivity is scarce. The findings regarding the potential biological effects of EMFs on redox homeostasis contradictory, with some investigations showing that EMFs induces oxidative stress and others showing no effects.12–15 These investigations utilized different parameters of EMFs, lacked measurements of insulin sensitivity and utilized no animal models of NAFLD. Thus, the bioeffect of EMFs on insulin resistance and oxidative stress has not been systematically assessed in NAFLD.
Here, we investigated the bioeffects of EMFs in db/db mice, which can recapitulate human NAFLD. Finally, we found that EMFs could improve IR and ameliorate hepatic oxidative distress and lipid accumulation in db/db mice. Therefore, we hypothesized that EMFs might attenuate the hepatic damage associated with NAFLD by modulating redox homeostasis. Thus, EMFs could be a novel treatment for NAFLD.
Methods and Materials
Animals and Experimental Design
Diabetic leptin receptor-mutant mice (db/db mice) were obtained from the Model Animal Research Centre of Nanjing University. The animals were housed in SPF conditions with 12-hour alternating light/dark cycles, and a constant temperature was maintained (22 °C). The animals were fed ad libitum. The animal experiments were approved by the Institutional Animal Care and Use Committee at Air Force Medical University and carried out according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Eight female C57BL/KsJ mice (18.07 ± 1.54 g) were used as controls. Sixteen female C57BL/6J db/db mice (29.73 ± 2.27 g) were equally and randomly divided into the db/db group and db/db + EMF group. The db/db + EMF group was exposed to electromagnetic fields (2 h/d, 1.6 mT). These parameters were in accordance with other published studies.16,17 The control and db/db groups were placed in identical cages with no EMF. Eight weeks after EMF exposure, all the mice were fasted overnight, and then, the fasting blood glucose levels were analysed. Liver tissues were harvested and stored at −80 °C before evaluation. Whole blood samples were centrifuged, and serum was collected for biochemical assays.
Electromagnetic Exposure System
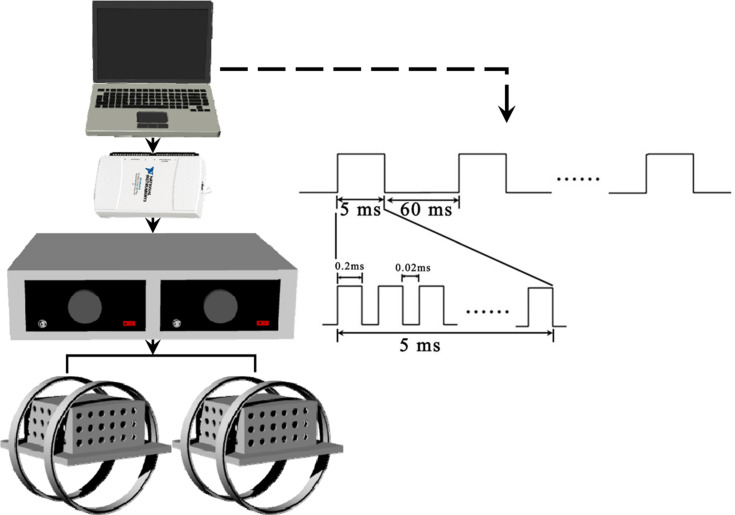
The EMF stimulators used in this study were described as previously reported.17,18 In brief, there were four parts: LabVIEW software, a multifunction data acquisition device (NI USB-6211), a power amplifier (XP9900S, Huamei, China) and Helmholtz coils. The waveform consisted of a pulse burst repeated at 15 Hz. The coils were 20 cm in diameter. Two copper enamelled round coils were placed 10 cm apart in parallel rows. A gaussmeter (Model 455, Lake Shore Cryotronics, USA) was used to accurately further confirm the distribution of the electromagnetic field intensity. In this study, the reference electromagnetic fields were set to 0 mT, and the intensity of the electromagnetic fields between coils was determined to be approximately 1.6 mT (mean ± SD, RMS). The background magnetic fields were to determined to be 50 ± 2 μT. A schematic representation of the EMF exposure system and output waveform is shown in Figure 1.
Figure 1.
Schematic representation of EMF exposure system and output waveform. The exposure waveform comprises a pulsed burst (burst width, 5 ms; pulse width, 0.2 ms; pulse wait, 0.02 ms; burst wait, 60 ms; pulse rise, 0.3 μs; pulse fall, 2.0 μs) repeated at 15 Hz.
Biochemical Analysis
Fasting blood glucose was analysed with a commercial blood glucose meter (OMRON Health Medical Co., Ltd., Japan), and serum insulin was measured using a mouse insulin ELISA kit (Cusabio Biotech Co., Ltd., Wuhan, China). The homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated by the following formula:
HOMA-IR = fasting insulin (m U/L) × fasting glucose (nmol/L)/22.5.9
Hepatic TG content, malondialdehyde (MDA), reduced glutathione (GSH), glutathione disulphide (GSSG), glutathione peroxidase (GSH-Px), catalase (CAT) and superoxide dismutase (SOD) assay kits were all purchased from Nanjing Jiancheng Bioengineering Institute (product numbers: A110-2-1, A003-1-2, A006-2-1, A061-1-1, A005-1-1, A007-2-1 and A001-3-2, respectively). The experimental procedures were performed according to the manufacturer’s instructions.19
Histological Analysis
Oil Red O reagent (Sigma-Aldrich Co., St. Louis, USA) were used to stain frozen liver slices to examine lipid accumulation within hepatocytes. Stained sections were detected with a light microscope and measured using Fiji software (ImageJ, NIH, USA).
Western Blot
Western blotting was used to examine protein expression, and the procedure was the same as that previously reported.19,20 In brief, HRP-conjugated goat anti-rabbit secondary antibodies (1:3000 diluted in TBST) were used. The protein bands were visualized by an ECL chemiluminescence reagent (Thermo Scientific, Rockford, IL, USA) following the manufacturer’s guidelines, and semiquantitative analysis was performed using Quantity One Software. β-Actin (1:2000, Bioworld Technology, Inc., Nanjing, China) was used as the internal control for normalization. The BCA Protein Assay Kit was purchased from Thermo Scientific (IL, USA). SREBP-1c antibody was purchased from Santa Cruz Biotechnology (1:1000, CA, USA). CAT antibody was purchased from Proteintech Group, Inc. (1:1000, Wuhan, China). Glutathione reductase (GR) and GSH-Px antibodies were purchased from Bioworld Technology, Inc. (1:1000, Nanjing, China). SOD1 and SOD2 antibodies were purchased from Abcam Biotechnology (1:1000, Cambridge, UK).
Statistical Analysis
All the data are expressed as the mean ± standard deviation (SD), and one-way ANOVA with an LSD t-test was used to determine the difference between two groups. Statistical analysis was performed with SPSS 16.0, and P < 0.05 was considered statistically significant.
Results
EMF Exposure Improved Insulin Resistance in db/db Mice
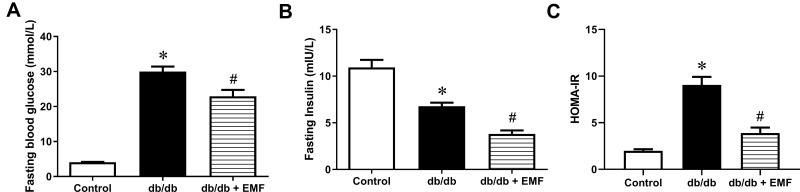
EMF exposure significantly improved fasting blood glucose and serum insulin in db/db mice (P < 0.05, Figure 2A and B). In the db/db mice, EMF exposure reduced the HOMA-IR by more than 50% compared with control (Figure 2C).
Figure 2.
Effect of EMF on IR in db/db mice. (A) Data of fasting blood glucose. (B) Data of fasting blood insulin. (C) Results of HOMA-IR. *P<0.05, compared with control; #P<0.05, compared with db/db group.
EMF Exposure Modulated Hepatic Redox in db/db Mice
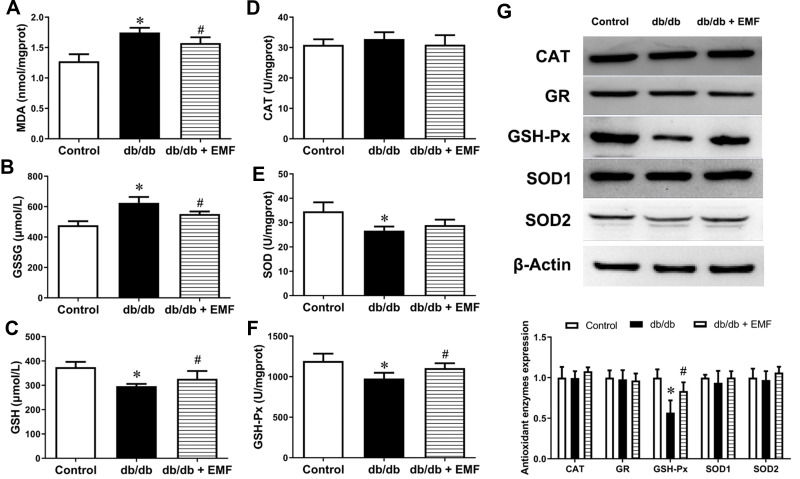
EMF exposure significantly elevated the reduced GSH level and lowered the MDA and GSSG levels in the db/db mice (P < 0.05, Figure 3A–C). In addition, EMF exposure increased the GSH-Px level (P < 0.05) but did not change the CAT and SOD levels (Figure 3D–F). The expression of the CAT, GR, SOD1 and SOD2 proteins was not changed by EMF exposure, whereas the overexpression of GSH-Px was observed to be significant (P < 0.05, Figure 3G).
Figure 3.
Effects of EMF exposure on redox homeostasis. (A) Liver MDA content. (B) Liver GSSG content. (C) Liver GSH content. (D) Activity/unit of CAT. (E) Activity/unit of SOD. (F) Activity/unit of GSH-Px. (G) Original recording and quantification of Western blotting. Values are all expressed as mean ± SD. *P<0.05, compared with control; #P<0.05, compared with db/db group.
EMF Exposure Ameliorated Hepatic Lipid Accumulation in db/db Mice
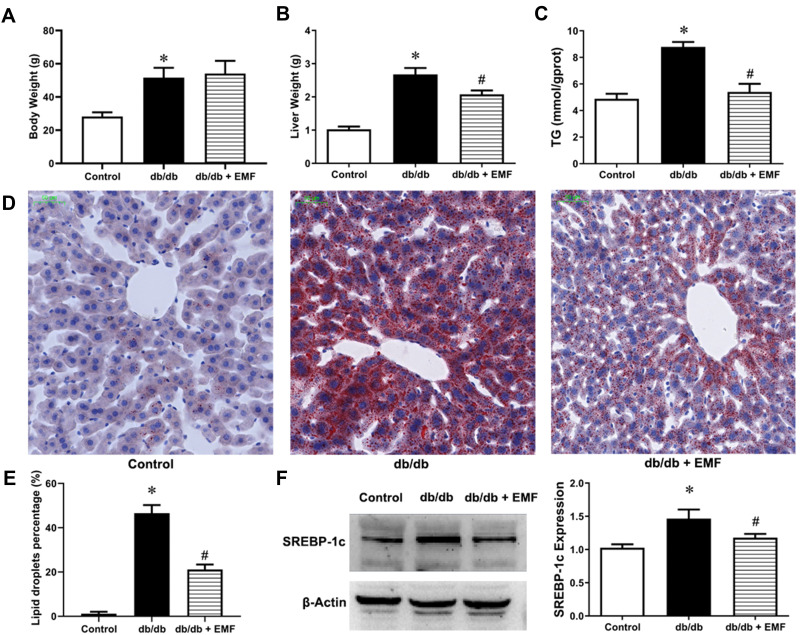
Body weight (Figure 4A) was not changed, but liver weight (Figure 4B) was significantly decreased by EMF exposure (P < 0.05). In addition, EMF exposure significantly reduced the liver TG content (Figure 4C). EMF exposure remarkably reduced the area of lipid droplets (P < 0.05, Figure 4D and E). The expression of the SREBP-1c protein was also significantly downregulated by EMF downregulated (P < 0.05, Figure 4F).
Figure 4.
Effects of EMF exposure on liver and SREBP-1c expression. (A) Weight of body. (B) Weight of liver. (C) Liver triglyceride content. (D) Oil Red O staining (×20). (E) Percentage of lipid droplets. (F) Liver SREBP-1c expression. Values are all expressed as mean ± SD. *P<0.05, compared with control; #P<0.05, compared with db/db group.
Discussion
NAFLD has become the most common form of chronic liver disease in the world and affects patients’ quality of life.21 The continuously growing prevalence of NAFLD is closely associated with T2DM, which is of great concern.22 To date, the pathogenesis of NAFLD has not been completely clarified, and there is no available treatment.1,3 Studies have shown that oxidative stress, as a key mechanism, has an impact on the progression of NAFLD by causing abnormal lipid metabolism.23–25 Accordingly, antioxidant therapy has become a potential treatment for NAFLD. Although very little is known, the biological responses caused by EMFs are allegedly mediated by the induction of interactions between quantum spin-state and paramagnetic radicals, which then regulate endogenous redox reactions.11,26,27 Accordingly, the present study explored the bioeffects of EMF exposure on redox homeostasis in a NAFLD animal model. We found that EMF exposure was associated with attenuated hepatic steatosis in db/db mice by improving insulin resistance and reducing oxidative stress.
Insulin resistance is defined as insensitivity to insulin and inability to dispose of blood glucose properly. Compared with wild-type mice, the db/db mice exhibited high blood glucose and a high index of HOMA-IR. This indicated that the db/db mice suffered from severe insulin resistance. Eight weeks of EMF treatment lowered fasting blood glucose, fasting serum insulin and the HOMA-IR index. These results suggested that EMFs could effectively improve impaired insulin sensitivity. These findings are similar to the results of a prior study.11 Studies have reported that redox imbalance deviating from oxidation contributes to the development of insulin resistance.28,29 MDA, as an indicator of oxidative stress, is an index of lipid peroxidation.30 We found that liver the MDA content increased sharply in db/db mice, which suggests hepatic oxidative stress injury. After treating db/db mice with EMFs, the MDA and GSSG levels were obviously decreased. In addition, EMF exposure strikingly increased the hepatic GSH levels, leading to a considerably reduced redox status in db/db mice. Moreover, adjusting the redox environment can alter insulin sensitivity and glycogen synthesis.29,31 Taken together, our results suggested that EMFs exert an insulin-sensitizing effect in part by regulating the systemic GSH/GSSG redox status.
To further explore the mechanisms of oxidative stress regulation through EMFs, a series of antioxidant enzyme activities were assessed. GSH, as a cellular reductant controller, is able to counteract oxidative damage by scavenging free radicals against oxidative damage.32 GSH-Px enhances the interaction between GSH and H2O2 to counteract peroxide.33 GSH-Px can transform GSH (reduced form of glutathione) as a cosubstrate into GSSG (oxidized form of glutathione). GSH-Px can remove hydrogen peroxide and scavenge lipid hydroperoxides to avoid peroxide damage.30,34 Subsequently, GR catalyses GSSG conversion into GSH. Low GSH levels weaken the antioxidant status and lead to lipid peroxidation enhancement.35 A case report showed that GSH-Px activity was significantly reduced, while the concentration of plasma MDA was obviously elevated in IR patients compared with controls.36 Recent studies have shown that increasing the activity of GSH-Px can inhibit oxidative stress in NAFLD rats with abdominal obesity.37 In our study, the activity/unit of CAT and SOD and the expression of CAT, SOD and GR were not altered. However, both the activity/unit and expression of GSH-Px increased obviously. Presumably, EMF exposure could improve hepatic oxidative stress in db/db mice by elevating the activity/unit and expression of GSH-Px and then promoting the antioxidant ability of enzymes.
Another important characteristic of NAFLD is excess lipid accumulation in hepatocytes. To date, recent studies have already shown that de novo lipogenesis strongly induces excess storage of TG in the liver, causing hepatic steatosis in NAFLD patients.38,39 Traditionally, it has been believed that abnormal hepatic lipid deposition causes mitochondrial dysfunction, which generates ROS and can result in oxidative stress.39,40 With increasing visceral adipose tissue mass, the risk of IR and metabolism disorders increases. Therefore, eliminating the abnormal accumulation of hepatic lipids might provide a way to treat metabolic disorders. This has been confirmed by several studies. Treating high-fat diet-fed mice with a mixture of Chinese herbal extracts can moderate IR, hyperlipidaemia and visceral obesity.41 Furthermore, daily intake of whey protein with a multimode exercise training programme can reduce fasting blood glucose and improve insulin sensitivity in overweight/obese people.42 We also focused on changes in the liver, the most important metabolic organ in the body, to determine the further beneficial bioeffects of EMFs on NAFLD. According to the liver weight, TG content and frozen sections stained with Oil Red O, the 8 weeks of EMF exposure obviously reduced the liver weight of db/db mice by alleviating lipid accumulation in hepatocytes. Prior research found that EMFs function similarly to physiological stress and can alter the lipid profile in the brain.43 The EMFs can also alter the lipid metabolism of soil nematodes.44 This study obtained analogous findings with the induction of positive effects on hepatic lipid accumulation by EMFs.
To explore the potential mechanism by which lipid metabolism is regulated by EMFs, we investigated sterol regulatory element binding protein-1c (SREBP-1c), which is a key transcription factor. An especially high level of SREBP-1c expression has been observed in fatty livers of obese, IR and hyperinsulinaemia animal models.45,46 We assessed the protein expression level of SREBP-1c because of the significant alleviation of hepatic lipid accumulation in db/db mice by EMFs. The results revealed that SREBP-1c expression could be downregulated significantly by EMFs. SREBP-1c expression is decreased by Meretrix oligopeptides to relieve the NAFLD induced by a high-fat diet.47 Xyloketal B can reduce SREBP-1c in NAFLD and decrease hepatic lipid accumulation.23 By inhibiting SREBP-1c expression in NAFLD, crude triterpenoid saponins from Ilex latifolia can also attenuate hepatic lipid accumulation.48 Therefore, the present study suggested that SREBP-1c expression was potentially downregulated and that abnormal liver lipid deposition could be reduced in db/db mice exposed to EMFs.
As with the majority of studies, the design of the current study was subject to the following limitations. (1) Although EMFs have been proven to be safe for orthopaedic applications, we need data to prove that EMFs are not harmful to the liver of wild-type mice. (2) Our research data assumed that EMF-mediated modification of SREBP-1c expression would continue, and we would need to clarify a plausible mechanism.
Conclusion
In conclusion, EMF exposure could increase antioxidant enzyme activity to improve IR and ameliorate lipid accumulation by downregulating SREBP-1c expression. Ameliorating the abnormal accumulation of lipids in the liver also improved impaired insulin sensitivity. Although further pathological mechanisms of NAFLD need to be elucidated and there is still no FDA-approved drug for NAFLD, this investigation provides a possible new solution for exploring NAFLD prevention and treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Younossi ZM, Loomba R, Rinella ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2018;68(1):361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. [DOI] [PubMed] [Google Scholar]
- 3.Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7(4):313–324. [DOI] [PubMed] [Google Scholar]
- 4.Sarwar R, Pierce N, Koppe S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metab Syndr Obes. 2018;11:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. [DOI] [PubMed] [Google Scholar]
- 7.Cichoz-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez A, Huerta-Salgado C, Orozco-Aguilar J, et al. Role of oxidative stress in hepatic and extrahepatic dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD). Oxid Med Cell Longev. 2020;2020:1617805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian YM, Liu Y, Wang S, et al. Anti-diabetes effect of chronic intermittent hypobaric hypoxia through improving liver insulin resistance in diabetic rats. Life Sci. 2016;150:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Elbatreek MH, Pachado MP, Cuadrado A, Jandeleit-Dahm K, Schmidt H. Reactive oxygen comes of age: mechanism-based therapy of diabetic end-organ damage. Trends Endocrinol Metab. 2019;30(5):312–327. [DOI] [PubMed] [Google Scholar]
- 11.Carter CS, Huang SC, Searby CC, et al. exposure to static magnetic and electric fields treats type 2 diabetes. Cell Metab. 2020;32(4):561–574.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai J, Zhang Y, Zhang J, et al. Effects of 100-muT extremely low frequency electromagnetic fields exposure on hematograms and blood chemistry in rats. J Radiat Res. 2016;57(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akdag MZ, Dasdag S, Ulukaya E, et al. Effects of extremely low-frequency magnetic field on caspase activities and oxidative stress values in rat brain. Biol Trace Elem Res. 2010;138(1–3):238–249. [DOI] [PubMed] [Google Scholar]
- 14.Patruno A, Tabrez S, Pesce M, et al. Effects of extremely low frequency electromagnetic field (ELF-EMF) on catalase, cytochrome P450 and nitric oxide synthase in erythro-leukemic cells. Life Sci. 2015;121:117–123. [DOI] [PubMed] [Google Scholar]
- 15.Li BL, Li W, Bi JQ, et al. Effect of long-term pulsed electromagnetic field exposure on hepatic and immunologic functions of rats. Wien Klin Wochenschr. 2015;127(23–24):959–962. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Lei T, Xie K, et al. Effects of extremely low frequency pulsed magnetic fields on diabetic nephropathy in streptozotocin-treated rats. Biomed Eng Online. 2016;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei T, Liang Z, Li F, et al. Pulsed electromagnetic fields (PEMF) attenuate changes in vertebral bone mass, architecture and strength in ovariectomized mice. Bone. 2018;108:10–19. [DOI] [PubMed] [Google Scholar]
- 18.Lei T, Li F, Liang Z, et al. Effects of four kinds of electromagnetic fields (EMF) with different frequency spectrum bands on ovariectomized osteoporosis in mice. Sci Rep. 2017;7(1):553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Zhai M, Guo F, et al. Whole-body vibration improves insulin resistance in db/db mice: amelioration of lipid accumulation and oxidative stress. Appl Biochem Biotechnol. 2016;179(5):819–829. [DOI] [PubMed] [Google Scholar]
- 20.Zhai M, Jing D, Tong S, et al. Pulsed electromagnetic fields promote in vitro osteoblastogenesis through a Wnt/beta-catenin signaling-associated mechanism. Bioelectromagnetics. 2016;37(3):152–162. [DOI] [PubMed] [Google Scholar]
- 21.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su ML, He Y, Li QS, Zhu BH. Efficacy of acetylshikonin in preventing obesity and hepatic steatosis in db/db mice. Molecules. 2016;21(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Meng T, Zuo L, et al. Xyloketal B attenuates fatty acid-induced lipid accumulation via the SREBP-1c pathway in NAFLD models. Mar Drugs. 2017;15(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66(1):180–190. [DOI] [PubMed] [Google Scholar]
- 25.Gentric G, Maillet V, Paradis V, et al. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J Clin Invest. 2015;125(3):981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiscock HG, Worster S, Kattnig DR, et al. The quantum needle of the avian magnetic compass. Proc Natl Acad Sci U S A. 2016;113(17):4634–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouritsen H. Long-distance navigation and magnetoreception in migratory animals. Nature. 2018;558(7708):50–59. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61(12):3208–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nocito L, Kleckner AS, Yoo EJ, et al. The extracellular redox state modulates mitochondrial function, gluconeogenesis, and glycogen synthesis in murine hepatocytes. PLoS One. 2015;10(3):e0122818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar AK, Vijayalakshmi K. Protective effect of Punica granatum peel and Vitis vinifera seeds on DEN-induced oxidative stress and hepatocellular damage in rats. Appl Biochem Biotechnol. 2015;175(1):410–420. [DOI] [PubMed] [Google Scholar]
- 31.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. [DOI] [PubMed] [Google Scholar]
- 32.Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther. 1991;51(2):155–194. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Luo Q, Cui H, et al. Sodium fluoride causes oxidative stress and apoptosis in the mouse liver. Aging (Albany NY). 2017;9(6):1623–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You BR, Shin HR, Han BR, Kim SH, Park WH. Auranofin induces apoptosis and necrosis in HeLa cells via oxidative stress and glutathione depletion. Mol Med Rep. 2015;11(2):1428–1434. [DOI] [PubMed] [Google Scholar]
- 35.Boysen G. The glutathione conundrum: stoichiometric disconnect between its formation and oxidative stress. Chem Res Toxicol. 2017;30(5):1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Chen H, Liu J, et al. Association between the NF-E2 related factor 2 gene polymorphism and oxidative stress, anti-oxidative status, and newly-diagnosed type 2 diabetes mellitus in a Chinese population. Int J Mol Sci. 2015;16(7):16483–16496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HY, Liang CM, Cui JW, et al. Acupuncture improves hepatic lipid metabolism by suppressing oxidative stress in obese nonalcoholic fatty liver disease rats. Zhen Ci Yan Jiu. 2019;44(3):189–194. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Hu J, Sheng L, et al. Ellagic acid ameliorates AKT-driven hepatic steatosis in mice by suppressing de novo lipogenesis via the AKT/SREBP-1/FASN pathway. Food Funct. 2019;10(6):3410–3420. [DOI] [PubMed] [Google Scholar]
- 39.Engin A. Non-alcoholic fatty liver disease. Adv Exp Med Biol. 2017;960:443–467. [DOI] [PubMed] [Google Scholar]
- 40.Satapati S, Kucejova B, Duarte JA, et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015;125(12):4447–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan Y, Kamal MA, Wang ZZ, et al. Chinese herbal extracts (SK0506) as a potential candidate for the therapy of the metabolic syndrome. Clin Sci (Lond). 2011;120(7):297–305. [DOI] [PubMed] [Google Scholar]
- 42.Arciero PJ, Baur D, Connelly S, Ormsbee MJ. Timed-daily ingestion of whey protein and exercise training reduces visceral adipose tissue mass and improves insulin resistance: the PRISE study. J Appl Physiol (1985). 2014;117(1):1–10. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Samano J, Flores-Poblano A, Verdugo-Diaz L, Juarez-Oropeza MA, Torres-Duran PV. Extremely low frequency electromagnetic field exposure and restraint stress induce changes on the brain lipid profile of Wistar rats. Bmc Neurosci. 2018;19(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Huang X, Wang Y, et al. Lipidomic alteration and stress-defense mechanism of soil nematode caenorhabditis elegans in response to extremely low-frequency electromagnetic field exposure. Ecotoxicol Environ Saf. 2019;170:611–619. [DOI] [PubMed] [Google Scholar]
- 45.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99(5):838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68(2):72–82. [DOI] [PubMed] [Google Scholar]
- 47.Huang F, Wang J, Yu F, et al. Protective effect of meretrix meretrix oligopeptides on high-fat-diet-induced non-alcoholic fatty liver disease in mice. Mar Drugs. 2018;16(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng RB, Fan CL, Liu Q, et al. Crude triterpenoid saponins from ilex latifolia (Da Ye Dong Qing) ameliorate lipid accumulation by inhibiting SREBP expression via activation of AMPK in a non-alcoholic fatty liver disease model. Chin Med. 2015;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]