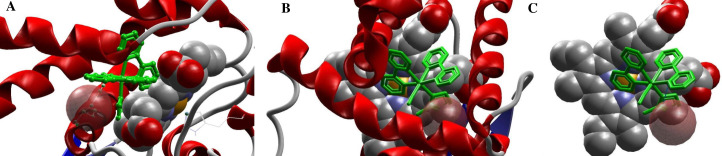
Fig 3. Interaction of the FOR811A compound, a ruthenium nitric oxide donor metallocompound with anti-asthmatic potential, on the Heme portion of the soluble guanylate cyclase (sGC) enzyme.
Through the exploration of the potential drug-protein binding mechanisms by the molecular docking method, it was observed that FOR811A bound strongly to the distal portion of the Heme group of sGC, specifically interacting with the residue Cys141 (a). In this study with H-NOX, FOR811A was shown to interact with the thiol portion of the cysteine residue at position 141 (Cys 141) (b, c).