Fig. 2. Intracellular mechanisms of action of MMF and fumarate prodrugs.
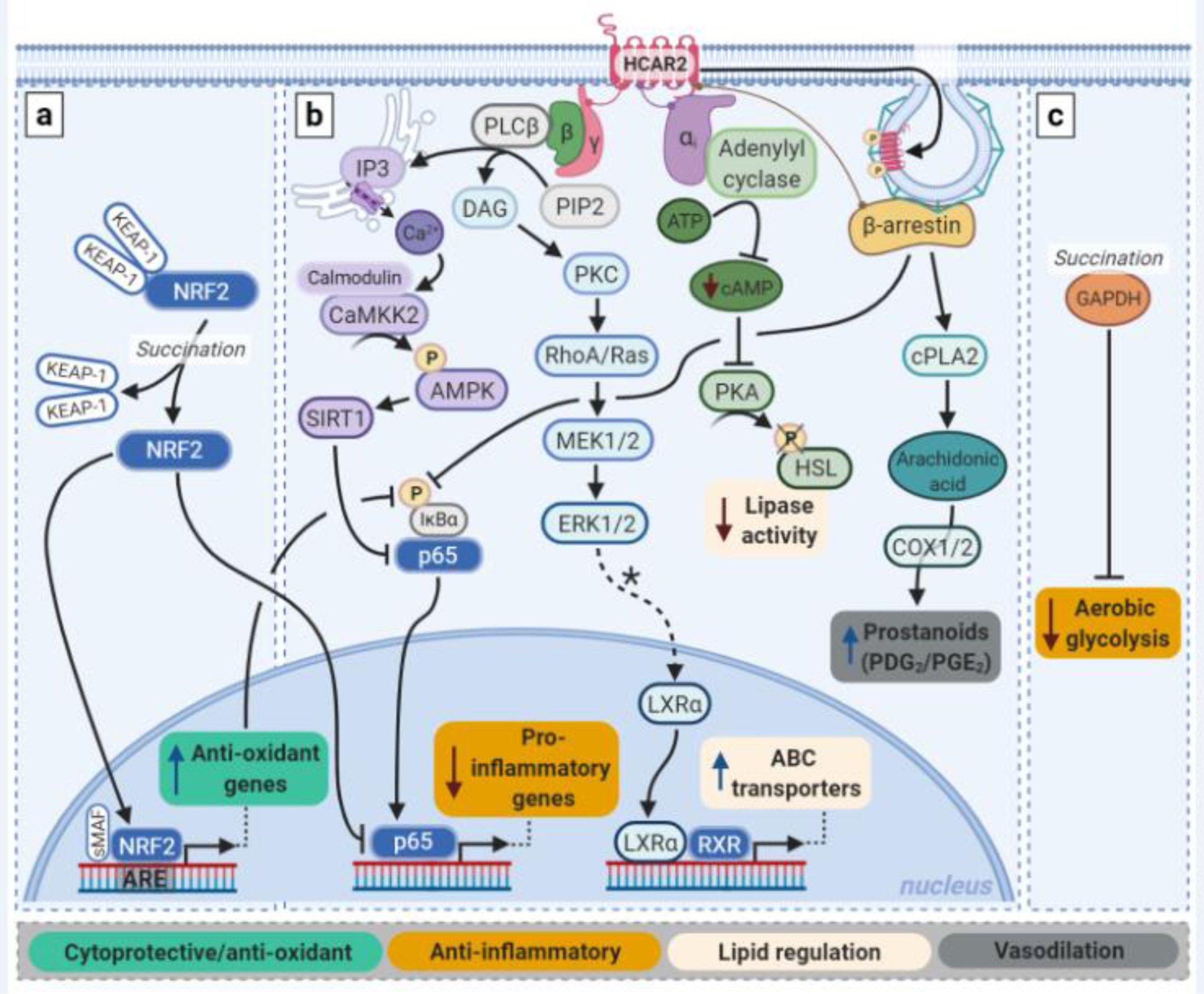
Fumarates impose their effects via three main pathways: a) the NRF2 pathway; b) the HCAR2 pathway; and c) via immunomodulation. a) Both MMF and fumarate prodrugs can induce activation and nuclear translocation of NRF2 by succination of Keap-1. Once inside the nucleus, NRF2 promotes transcription of cytoprotective genes, including those encoding antioxidants. The pro-inflammatory transcription factor NFkB can be inhibited by the NRF2 pathway both via a reduction in ROS levels and via competition with NRF2 over CREB-binding proteins. b) MMF is a full agonist of HCAR2. Activation of this Gi-protein coupled receptor results in release of the Giα and Gβƴ proteins. This leads to inhibition of NFkB activation and lipase activity. Via PKC and ERK1/2, PPARƴ-mediated transcription of LXRα is thought to be upregulated, which in turn promotes transcription of ABC transporters. β-arrestin binding to HCAR2 is important in receptor regulation by promoting desensitization and internalization via clathrin-coated pits, and it can inhibit NFĸB activity. β-arrestin is also involved in prostanoid production mediated by cyclo-oxygenase (COX) 1/2 in keratinocyte and Langerhans cells. c) Independent of the NRF2 and HCAR2 pathways, MMF and prodrugs can reduce inflammation by blocking glycolytic metabolism via succination and thus inactivation of GADPH, which favors differentiation of macrophages and T-cells to an anti-inflammatory phenotype. In general, of note, the pharmacodynamics of MMF are likely to be most clinically relevant. PIP2: phosphatidylinositol biphosphate; DAG: diacylglycerol; ATP: adenosine triphosphate; HSL: hormone-sensitive lipase. *Hypothesized.
