Table 1.
Pharmacokinetic properties of fumarate drugs evaluated in humans.
| Drug | Approval | Indication | Dosing regime | MMF equiv. | MMF T1/2 (h) | MMF Tmax (h) | MMF Cmax (mg/l) | MMF AUC0−∞ (h.mg/l) | MMF Vd (l) | MMF %PPB | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fed | fasted | fed | fasted | fed | fasted | fed | fasted | ||||||||
Fumaderm®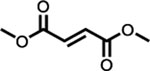 (dimethyl fumarate) (salts of monoethyl fumarate) |
German Drug Administration (1994) | Psoriasis | Single dose 2 × 120 mg tablet (total 240 mg)a,d | 216 mg | 0.65 | 3.5 | 1.46 | 2.87 | [7] | ||||||
Tecfidera®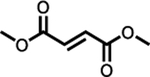 (dimethyl fumarate) |
EMA (30st January 2014); FDA (27th March 2013) | MS | Single dose 240 mgb | 216 mg | 1 | 5.0 | 2.0–2.5 | 1.87 | 3.21 (reported as 40% increase from fed) | 8.21e (4.11) | Equivalent to fed | 53–73 | 27–45 | [131] | |
| Single dose 240 mgc | 216 mg | 1.28 | 3.0 | 1.83 | 3.67 | [132] | |||||||||
| 240 mg tablet BIDc | 432 mg | 2.17f | 7.01g | [133] | |||||||||||
Skilarence®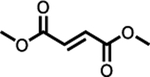 (dimethyl fumarate) |
EMA (23rd June 2017) | Psoriasis | 120 mg tablet once dailya | 108 mg | 9.0 | 3.5 | 1.311 | 1.325 | 50 | [134] | |||||
Vumerity™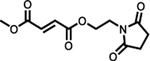
|
FDA (29th October 2019) | MS | Single dose 462 mgb | 235 mg | 1 | 7.0 | 2.5–3.0 | 1.18 (reported as 44% decrease from fasted) | 2.11 | Equivalent to fasted | 8.32e (4.16) | 72–83 | 27–45 | [135] | |
| Single dose 462 mgc | 235 mg | 0.76 | 2.5 | 1.70 | 3.81 | [132] | |||||||||
Bafiertam™ (monometh yl fumarate) |
FDA (28th April 2020) | MS | Single dose 2 × 95 mg tablets (total 190 mg)b | Figure | 0.5 | 11 | 4.03 | Reported as decreased by 20% from fasted | Bioequivalent to Tecfidera 240 mg (216 mg MMF equiv.) | Bioequivalent to Tecfidera 240 mg (216 mg MMF equiv.) | 53–73 | 27–45 | [107] | ||
| Tepilamide
fumarate |
Phase 2 clinical trials | Psoriasis | 200 mg tablet BIDc | 214 mg | 1.49f | 3.67g | [133] | ||||||||
| 400 mg tablet BIDc | 428 mg | 2.20f | 6.91g | [133] | |||||||||||
Moderate to severe plaque psoriasis patients.
Relapsing-remitting multiple sclerosis patients.
Healthy subjects.
Each 120 mg tablet also contains 95 mg of a mixture of MEF salts.
AUC0−∞ after intake twice a day (BID)
Reported as Cmax,ss
Reported as AUC24,ss. Tmax (time of maximal concentration), Cmax (maximal concentration), AUC (area under the curve, drug concentration in blood plasma as a function of time), Vd (volume of distribution), PPB (plasma protein binding).
