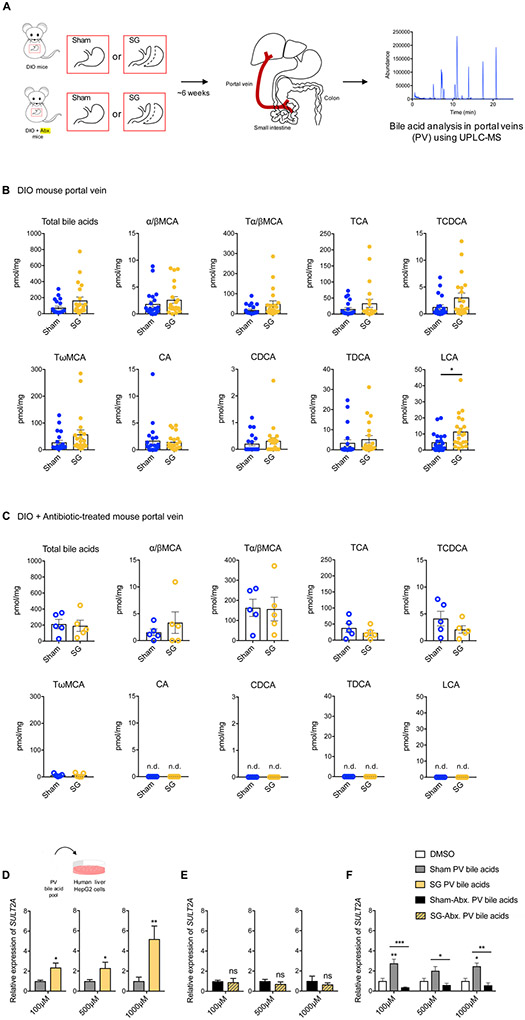
Figure 2. Portal vein BAs induce expression of hSULT2A1 in hepatocytes in vitro.
(A) Schematic of portal vein BA analysis in indicated groups of mice.
(B) Portal vein BAs in DIO mice 6 weeks post-Sham and SG (n=21 in each group; data not marked with asterisk(s) are not significant). All bile acids with measurable concentrations above the limit of detection are shown. Lithocholic acid (LCA) was the only bile acid whose levels were significantly increased post-SG (*p=0.01). (Total bile acids, p=0.51, α/βMCA, alpha-muricholic acid and beta-muricholic acid, p=0.35, Tα/βMCA, tauro-alpha- and tauro-beta-muricholic acid, p=0.08; TCA, tauro-cholic acid, p=0.20; TCDCA, tauro-chenodeoxycholic acid, p=0.07; TωMCA, tauro-omega-muricholic acid, p=0.11; CA, cholic acid, p=0.78; CDCA, chenodeoxycholic acid, p=0.45; TDCA, tauro-deoxycholic acid, p=0.45; Welch’s t test).
(C) Portal vein BAs in DIO mice treated with antibiotics post-sham and SG (n=5 in each group; data not marked with asterisk(s) are not significant. LCA, CA, TDCA, and CDCA were undetectable in portal veins of both groups. (Total bile acids, p=0.81, α/βMCA, alpha-muricholic acid and beta-muricholic acid, p=0.43; Tα/βMCA, tauro-alpha- and tauro-beta-muricholic acid, p=0.93; TCA, tauro-cholic acid, p=0.36; TCDCA, tauro-chenodeoxycholic acid, p=0.23; TωMCA, tauro-omega-muricholic acid, p=0.98; CA, cholic acid; CDCA, chenodeoxycholic acid; TDCA, tauro-deoxycholic acid; LCA, lithocholic acid, not detected (n.d.), Welch’s t test).
(D-F) Concentrated pools of bile acids mimicking the mean physiological ratios of individual portal vein bile acids measured in conventional sham and SG and antibiotic-treated sham and SG mice were generated in vitro in DMSO. HepG2 cells were treated with dilutions of these pools (total bile acid concentrations of 100 μM, 500 μM, and 1000 μM). (D) As measured by qRT-PCR, the expression of hSULT2A was increased in conventional SG PV BA-treated cells compared to conventional sham PV BA-treated cells. For each concentration, expression of sham hSULT2A was normalized to 1. hSULT2A expression was normalized to human GAPDH (≥3 biological replicates per condition, SG PV bile acids, 100 μM *p=0.01, 500 μM *p=0.02, 1000 μM **p=1.00x10−3, Welch’s t test). (E) There were no significant differences in hSULT2A expression in cells incubated with Abx-treated SG and sham PV BA pools. For each concentration, expression of sham hSULT2A was normalized to 1. hSULT2A expression was normalized to human GAPDH (≥3 biological replicates per condition, SG-Abx. PV bile acids, ns=not significant, 100 μM p=0.82, 500 μM p=0.37, 1000 μM p=0.60, Welch’s t test). (F) As measured by qRT-PCR, the expression of hSULT2A was increased in conventional sham PV BA-treated cells and decreased in antibiotic sham PV BA-treated cells relative to DMSO control. hSULT2A expression was normalized to human GAPDH (≥3 biological replicates per condition, data not marked with asterisk(s) are not significant, 100 μM DMSO vs. Sham **p=5.60x10−3; 100 μM DMSO vs. Sham-Abx. p=0.82; 100 μM Sham vs. Sham-Abx. ***p=1.00x10−4; 500 μM DMSO vs. Sham p=0.29; 500 μM DMSO vs. Sham-Abx. p=0.98; 500 μM Sham vs. Sham-Abx. *p=0.04; 1000 μM DMSO vs. Sham *p=0.03; 1000 μM DMSO vs. Sham-Abx. p=0.97; 1000 μM Sham vs. Sham-Abx. **p=2.30x10−3, two-way ANOVA followed by Dunnett’s multiple comparisons test).
All data are presented as mean ± SEM.