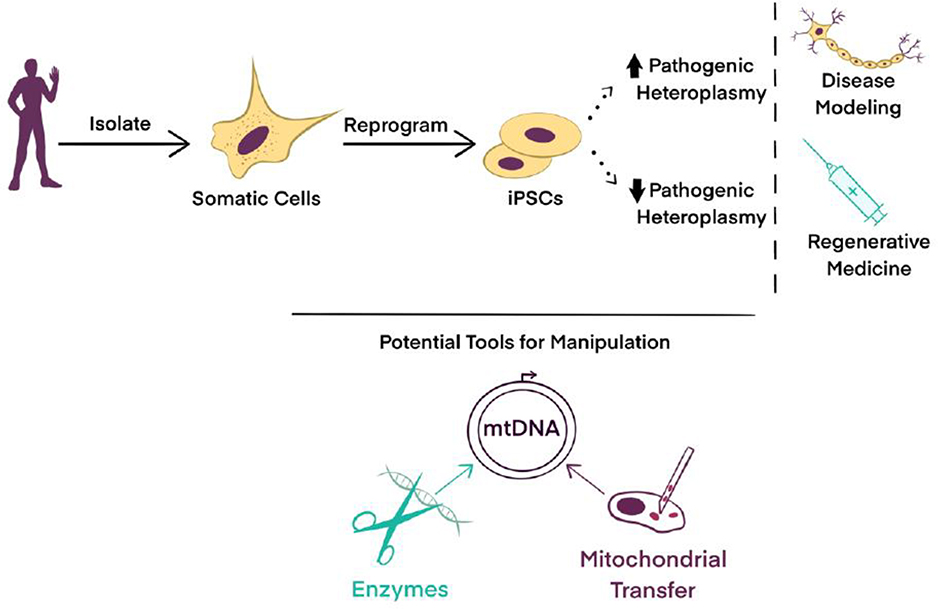
Figure 4.
Controlling Heteroplasmy in iPSCs for Clinical Applications. The utility of reprogramming patient-derived somatic cells into iPSCs may become limited for many reasons including the uncontrolled generation of suboptimal mtDNA heteroplasmy ratios. Tools and approaches have been developed to controllably manipulate the mtDNA content in somatic cells that can then be converted to iPSCs, or in iPSCs themselves, including targeted endonucleases, somatic cell nuclear transfer, and engineered mitochondrial transfer modalities. Methodologies to specify heteroplasmy levels in iPSCs could improve applications in disease modeling, drug screening, and regenerative medicine by tailoring mtDNA populations for specific applications.