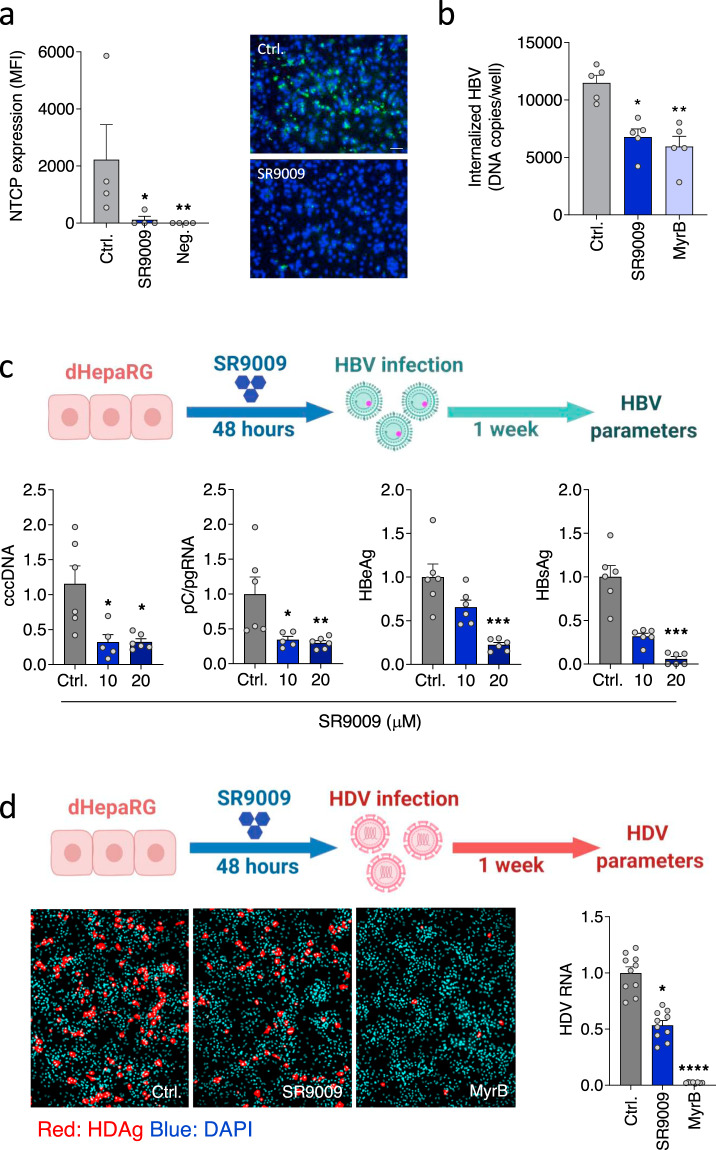
Fig. 3. Pharmacological activation of REV-ERB inhibits HBV and HDV infection.
a dHepaRG cells were treated with SR9009 (20 µM, 48 h) and NTCP expression assessed using Myrcludex B (MyrB) tagged with Alexa 488 fluorophore. Unstained cells were used as the negative control. Median fluorescence intensity was quantified (mean ± SEM, n = 4, Kruskal–Wallis ANOVA with Dunn’s test). Representative fluorescent images are shown (scale bar = 100 µm). b dHepaRG cells were treated with SR9009 (20 µM) or MyrB (200 nM) and inoculated with HBV at 4 °C for 3 h. Unbound virus was removed by washing and the inoculated cells incubated at 37 °C for 16 h, trypsinized to remove non-internalized virus and intracellular HBV DNA levels quantified by qPCR and presented as copies per well (mean ± SEM, n = 5, Kruskal–Wallis ANOVA with Dunn’s test). c dHepaRG cells were treated with SR9009 (10 or 20 µM) for 48 h and inoculated with HBV for 6 h. 1 week post-infection, cccDNA, pC/pgRNA, HBeAg and HBsAg were quantified by qRT-PCR or ELISA assay. In all cases, data are expressed relative to untreated (Ctrl) cells (mean ± SEM, n = 6, Kruskal–Wallis ANOVA with Dunn’s test). d dHepaRG cells were treated with SR9009 (20 µM) for 48 h and inoculated with HDV and 7 days post-infection, HDV antigen (HDAg) and viral RNA measured by fluorescent staining or qRT-PCR. MyrB was included as the positive control to assess NTCP-dependent HDV infection (mean ± SEM, n = 8–10, Kruskal–Wallis ANOVA with Dunn’s test). *p < 0.05, **p < 0.01, ***p < 0.001. Data are provided in the accompanying Source Data file.