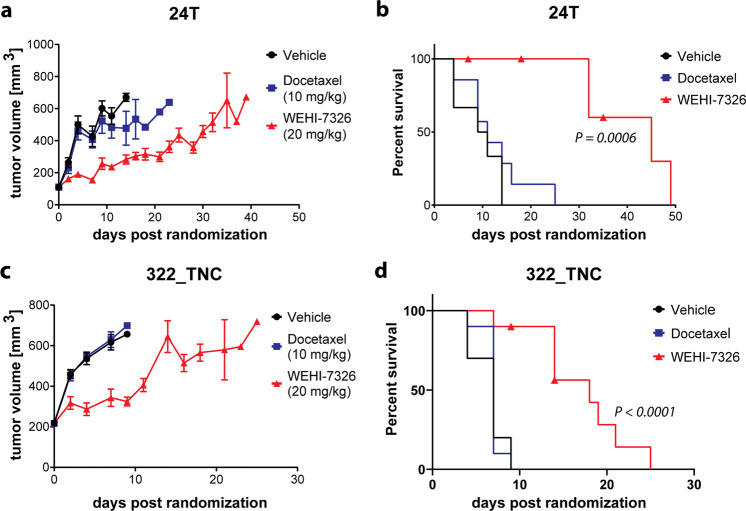
Fig. 4. Efficacy of WEHI-7326 in taxane-refractory patient-derived xenograft mouse models of triple-negative breast cancer.
a, b Tumor growth curve (a) and Kaplan–Meier survival plot (b) of 24 T TNBC PDX recipient female mice treated with either docetaxel (10 mg/kg, i.p.; once) or WEHI-7326 (20 mg/kg, i.v.; twice a week). p(docetaxel) = 0.3307 (ns), p(WEHI-7326) = 0.0006 (***) compared to vehicle control; p-values obtained through Log-rank (Mantel-Cox) test, n = 7 mice. Adverse effects in WEHI-7326 arm: 1 sick, 1 found dead, 1 with back leg paralysis. c, d Tumor growth curve (c) and Kaplan–Meier survival plot (d) of 322 T TNBC PDX recipient female mice treated with either docetaxel (10 mg/kg, i.p.; once) or WEHI-7326 (20 mg/kg, i.v.; twice a week). Vehicle mice were administered the vehicle for WEHI-7326. p(docetaxel) = 0.7974 (ns), p(WEHI-7326) <0.0001 (****) compared to vehicle control; p-values obtained through Log-rank (Mantel-Cox) test, n = 10 mice. Adverse effect in WEHI-7326 arm: 1 sick, 1 found dead.