Abstract
Despite many improvements in its prevention and management, acute coronary syndrome (ACS) remains a major cause of morbidity and mortality in the developed world. Lipid management is an important part of secondary prevention after ACS, but many patients currently remain undertreated and do not attain guideline‐recommended levels of low‐density lipoprotein cholesterol reduction. This review details the current state of evidence on lipid management in patients presenting with ACS, provides directions for identification of patients who may benefit from early escalation of lipid‐lowering therapy, and discusses novel lipid‐lowering medication that is currently under investigation in clinical trials. Moreover, a treatment algorithm aimed at attaining guideline‐recommended low‐density lipoprotein cholesterol levels is proposed. Despite important advances in the initial treatment and secondary prevention of ACS, ≈20% of ACS survivors experience a subsequent ischemic cardiovascular event within 24 months, and 5‐year mortality ranges from 19% to 22%. Knowledge of the current state of evidence‐based lipid management after ACS is of paramount importance to improve outcomes after ACS.
Keywords: acute coronary syndrome, cardiovascular, lipids
Subject Categories: Cardiovascular Disease, Secondary Prevention, Risk Factors
Nonstandard Abbreviations and Acronyms
- AEGIS‐1
apo‐I event reducing in ischemic syndromes I
- EPA
eicosapentaenoic acid
- PCSK9
proprotein convertase subtilisin‐kexin type 9
Despite many improvements in its prevention and management, coronary artery disease (CAD) remains a major cause of death in the developed world. 1 Acute coronary syndrome (ACS) constitutes the most severe clinical manifestation of CAD and includes unstable angina, non–ST‐segment–elevation myocardial infarction (MI), and ST‐segment–elevation MI. Recent data from the National Heart, Lung, and Blood Institute suggest that the annual incidence of MI in the United States is 805 000. 2 The treatment of ACS has progressed tremendously since the 1950s and 1960s when it was associated with in‐hospital mortality as high as 30%. 3 By determining the underlying pathophysiological features and conducting large‐scale randomized controlled trials, the management of ACS evolved and in‐hospital mortality was reduced to ≈3% to 8%. 4 Nonetheless, in the current era, ≈20% of ACS survivors experience a subsequent ischemic cardiovascular event within 24 months, and 5‐year mortality ranges from 19% to 22%. 5 , 6 Even when nonemergent and uncomplicated, repeated revascularization following percutaneous coronary intervention has been associated with long‐term mortality. 7 , 8 , 9 A recurrent ACS is associated with increased mortality to an even greater extent. 10 There are limits to the amount of antithrombotic medication that can be prescribed for secondary prevention, as increased intensity of antithrombotic therapy decreases recurrent ischemic events at the cost of increased bleeding events, which are also associated with subsequent mortality. 10
As lipids play a critical role in the development of coronary atherosclerosis lesions, obtaining a significant reduction of the lipid‐related risk has long been a crucial aspect of secondary prevention following ACS. Several additions to the available arsenal of lipid‐lowering therapies have recently been made or will soon be made, leaving clinicians with various therapeutic strategies to be used according to the clinical setting. This review details the current state of evidence on lipid management in patients presenting with ACS, provides directions for identification of patients who may benefit from early escalation of lipid‐lowering therapy, and discusses novel lipid‐lowering medication that is currently under investigation in clinical trials.
Current Insights and Recommendations on Lipid‐Lowering Therapy After ACS
Large‐scale trials are ongoing to investigate the clinical relevance of lipid particles, such as triglycerides, high‐density lipoprotein cholesterol (HDL‐C), apolipoprotein A1, and lipoprotein(a), in reducing the persistent risk of developing CAD and its complications. However, increased levels of low‐density lipoprotein cholesterol (LDL‐C) have irrefutably been shown to be a key causal factor in the development of CAD, and robust clinical evidence shows that reducing LDL‐C blood levels leads to the prevention of atherothrombotic events. 11 , 12 Figure 1 provides an overview of the distinct mechanisms of action for the 3 classes of cholesterol‐lowering drugs that are being advocated in current guidelines (ie, statins, ezetimibe, and PCSK9 [proprotein convertase subtilisin‐kexin type 9] inhibitors).
Figure 1. Major mechanisms of action of statins, ezetimibe, and PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors.
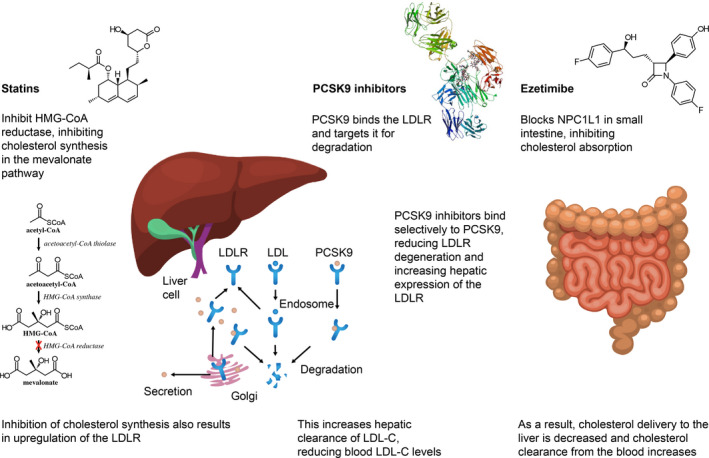
HMG CoA indicates hydroxy‐3‐methyglutaryl coenzyme A; LDL, low‐density lipoprotein; LDL‐C, LDL cholesterol; LDLR, LDL receptor; and NPC1L1, Niemann‐Pick C1 like.
The more intensive the lowering of LDL‐C, the greater the benefit in terms of reducing atherothrombotic events. A large‐scale meta‐analysis of almost 170 000 patients, most of whom presented with a documented coronary heart disease, from 26 trials comparing statins versus placebo or high‐intensity versus low‐intensity statins reported that each 1.0‐mmol/L reduction (≈39 mg/dL) of LDL‐C results in a 20% relative reduction of the annual rate of adverse events, including coronary death, nonfatal MI, coronary revascularization, and ischemic stroke. 12 This meta‐analysis included trials in the setting of primary and secondary prevention, with most patients (59%) being treated for secondary prevention. Interestingly, there was no evidence of a threshold below which further reduction of LDL‐C no longer resulted in additional benefit. Evidence from studies investigating PCSK9 inhibitors showed sustained reduction of adverse atherothrombotic events at low levels of LDL‐C (<40 mg/dL or <1 mmol/L), with a favorable safety profile. 13 , 14 A meta‐regression analysis of 312 175 patients from 49 randomized trials investigating statin and nonstatin therapies for primary or secondary prevention reported similar risk reduction per change in LDL‐C for both therapies, with a relative risk of 0.77 (95% CI, 0.71–0.81) and 0.75 (95% CI, 0.66–0.86) per 1‐mmol/L reduction in LDL‐C levels, respectively. 15 This is an important finding, as statins are thought to provide additional beneficial effects on top of LDL‐C lowering because of their anti‐inflammatory pleiotropic properties. 16 Data on potential pleiotropic effects of PCSK9 inhibitors remain scarce but are emerging. Although PCSK9 inhibitors do not reduce CRP (C‐reactive protein) levels, experimental research reports an association between higher PCSK9 plasma levels, high on‐treatment platelet reactivity, and elevated factor VIII levels. 17 Reduced PCSK9 function has been associated with decreased sepsis‐related inflammatory response and improved outcomes in murine models and humans. 18 Ongoing studies are investigating the potential impact of PCSK9 inhibitors on platelet reactivity (NCT03096288) and sepsis‐related inflammation and outcomes (NCT03634293). Finally, PCSK9 inhibitors may also provide additional pleiotropic beneficial effects related to lowering of lipoprotein(a). 19
Current Guideline Recommendations
Both the American Heart Association/American College of Cardiology guideline on the management of blood cholesterol and the European Society of Cardiology guidelines for the management of dyslipidemias recommend obtaining a lipid profile after 4 weeks of admission for ACS. 20 , 21 As LDL‐C levels vary minimally after normal food intake, a nonfasting sample can be used. 22 An overview of current American Heart Association/American College of Cardiology guideline recommendations for lipid‐lowering therapy applied to patients with ACS is shown in Figure 2. In brief, 3 agents with well‐documented safety and efficacy can be prescribed (statins, PCSK9 inhibitors, and ezetimibe); the appropriate timing of initiation and/or escalation of these agents depends on (1) whether patients are already on maximally tolerated doses of statin and/or ezetimibe and (2) the LDL‐C level at the time of ACS. The foundation of LDL‐C–lowering therapy is the prompt initiation of high‐intensity statin, followed by the addition of either ezetimibe or PCSK9 inhibitors to hopefully blunt the short‐term recurrent ischemic complication rate. Guidelines advocate adding ezetimibe first as this is a more cost‐effective strategy, but also allow for initiation of PSCK9 inhibitors without ezetimibe, as outlined in Figure 2. The rationale for considering initiation of a PSCK9 inhibitor without first starting ezetimibe is that only 3% and 5% of patients were on ezetimibe in the large phase 3 randomized FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) and ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) trials, respectively, which demonstrated the clinical benefit of PCSK9 inhibitors for secondary prevention of atherosclerotic cardiovascular disease (ASCVD). 23 , 24 Moreover, the expected additional reduction in LDL‐C with PCSK9 inhibitors when added to statins is ≈60%, 23 whereas ezetimibe is only 24%. 25 The number needed to treat to prevent an ischemic end point in the ODYSSEY OUTCOMES trial with alirocumab was ≈63 in just under 3 years of medical follow‐up and was 50 over 7 years with ezetimibe. 24
Figure 2. Current American Heart Association/American College of Cardiology guideline recommendations for lipid‐lowering therapy in patients with acute coronary syndrome (ACS).

*Recommendations for very‐high‐risk patients, defined as a history of multiple atherosclerotic cardiovascular disease events or 1 major atherosclerotic cardiovascular disease event and multiple high‐risk conditions, including aged ≥65 years, heterozygous familial hypercholesterolemia, diabetes mellitus, hypertension, smoking, and history of congestive heart failure. †High‐intensity statin includes atorvastatin, 40 to 80 mg/d, or rosuvastatin, 20 to 40 mg/d. LDL‐C indicates low‐density lipoprotein cholesterol; and PCSK9, proprotein convertase subtilisin/kexin type 9.
Adverse Effects of Lipid‐Lowering Medication and Potential Concerns With Low Blood LDL‐C Concentrations
The most important adverse effects of statins include myalgias and elevated liver enzymes (occurring in 0.5%–3.0% of patients), whereas clinically significant hepatic injury is rare and likely has an incidence no different from that in the general population. 26 Moreover, statins may confer a small increased risk of increasing plasma glucose levels and developing diabetes mellitus, particularly in a prediabetic patient; however, the totality of the available clinical evidence suggests that their beneficial effects outweigh this potential detrimental impact. 27 In fact, a pooled analysis of data from 5 trials, including mainly patients presenting with prior MI, reported that the use of high‐intensity, compared with moderate‐intensity, statins was associated with new onset of diabetes mellitus in 1 case per year for 498 patients treated, whereas the same regimen prevented 1 cardiovascular event per year for 155 patients treated. 28 Of note, the risk for developing new onset of diabetes mellitus in patients treated with high‐intensity statins increases with the presence of each component of the metabolic syndrome (ie, body mass index, hypertension, fasting triglycerides level, and blood glucose). 29 Other potential associations between statins and adverse events, such as intracranial hemorrhage and an increased risk of cancer, were not substantiated in large‐scale meta‐analyses of randomized controlled trials. 12 The PCSK9 inhibitors alirocumab and evolocumab are well tolerated, with pooled clinical trial data showing that overall rates of adverse effects were similar to placebo. 13 , 30 The most common adverse effects include mild local injection‐site reactions (eg, bruising, erythema, or pain). Current evidence from large‐scale randomized trials investigating secondary prevention of ASCVD suggests that aggressively lowering LDL‐C to very‐low levels (<25 mg/dL) does not result in unanticipated adverse events. 23 , 31 Because of postmarketing safety concerns of mild and reversible cognitive impairment associated with statins, there has been considerable effort to investigate possible neurocognitive impairment with PCSK9 inhibitors. 32 , 33 The EBBINGHAUS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects study investigated neurocognitive function in 1204 patients from the FOURIER trial who were randomized to evolocumab or placebo over a median period of 19 months and showed no difference in cognitive function. 31 , 34 Nonetheless, some caveats apply as the very‐low LDL‐C levels attained with PCSK9 inhibitor therapy bring us into hitherto‐uncharted territory; longer‐term data on their safety, such as the planned extension of EBBINGHAUS study up to 5‐year follow‐up (NCT02867813), are eagerly anticipated.
Unmet Need in Contemporary Clinical Practice
Despite the established benefit of lowering LDL‐C in patients with recent ACS, there remain important treatment gaps on the achievement of guideline‐recommended LDL‐C targets, a phenomenon that has been observed in the United States as well as other industrialized countries. 35 , 36 , 37 , 38 , 39 , 40 , 41 Reasons for this undertreatment are multiple and include adverse effects and perceived risks of statins; racial, sex, and geographical disparities; variations in protocols and practices across health systems; perceived prohibitive costs; and clinical inertia in cases of preexisting lipid‐lowering medications (Figure 3). Potential strategies to facilitate achieving guideline‐directed LDL‐C targets are listed below.
Figure 3. Factors influencing the inability to lower low‐density lipoprotein cholesterol (LDL‐C) to guideline‐recommended targets.

Clinical Inertia
Physicians may be reluctant to alter long‐standing and apparently well‐tolerated medication regimens. Continuing medical education is important to keep physicians up to date about new guideline‐directed treatment targets and medication strategies, and could potentially also address barriers to prescription of novel drugs, including patient copays and coverage issues by insurers.
Medication Nonadherence
The exact prevalence of medication nonadherence is difficult to estimate, but is an important modifiable problem. 42 Real‐world data suggest that statin discontinuation rates may be as high as 59.2% at 12 months, with only approximately half of patients being rechallenged within the subsequent 12 months. 43 A 10% reduction in the statin medication possession ratio is associated with a 5% increased risk for cardiovascular disease‐related hospitalizations. 44 Reasons for medication nonadherence are multifactorial, and are influenced by socioeconomic factors (eg, copays and insurance issues), concomitant illnesses, and therapy‐related factors (eg, adverse effects or frequent dose changes). 45 More than half of patients eligible for statin therapy in the PALM (Patient and Provider Assessment of Lipid Management) Registry, but who were not on treatment, reported never having been offered a statin by their physician. Concern about adverse effects was the leading reason for statin refusal or discontinuation. Many patients were willing to reconsider statin therapy if offered. 46 Therefore, a multidisciplinary approach is needed to increase adherence aimed at knowledge dissemination, improved patient engagement strategies, alleviating health disparities, and optimizing physician‐patient communication.
Racial, Sex, and Geographical Disparities
Racial and ethnic minorities in the United States experience a higher overall prevalence of risk factors for ASCVD that often go unrecognized and/or untreated. 47 , 48 This is particularly important as it is expected that, within several decades, non‐Hispanic White individuals will no longer form the majority of the US population. Similarly, female sex is still associated with a higher likelihood of not being prescribed statins or not achieving guideline‐directed LDL‐C levels. 37 , 48 Therefore, physicians should be educated in “cultural competency,” which includes weighing diverse values, beliefs, and behaviors to meet patients' social, cultural, and linguistic needs. 49 Geographical disparities are also well documented, in both non‐Hispanic White individuals and minorities. 50 , 51 Patients living in remote areas with limited access to healthcare facilities may benefit in the future from telemedicine applications.
Cost Barriers
Cost barriers may include out‐of‐pocket costs for patients or high drug costs for payers. Statins are presently available to most Americans at little to no out‐of‐pocket cost, which has previously been associated with increased prescription fills for statin therapy. 52 PCSK9 inhibitors are costly drugs whose cost‐effectiveness may be improved by selecting patients at (very) high risk of ASCVD events. 53
Adverse Effects
Although evidence from placebo‐controlled, randomized controlled trials shows that there are relatively low rates (5%–10%) of statin discontinuation because of adverse effects, 26 they are one of the most common reasons for statin discontinuation. 43 , 54 Nonetheless, most patients who are rechallenged are able to tolerate long‐term statins, highlighting the importance of statin rechallenging. 43 The adverse effect profile of PCSK9 inhibitors seems to be rather favorable compared with that of statins, and these drugs may therefore be an alternative in truly statin‐intolerant patients. 55
Variations in Treatment Protocols Across Health Systems
Health system level interventions may facilitate achieving guideline‐recommended LDL‐C targets, and may also accelerate the lag between publication of guidelines and their clinical implementation. Regular audits and providing feedback to providers may help to close the gap between research and practice. 56 Moreover, implementation of treatment algorithms may be helpful to streamline decision‐making toward achieving optimal LDL‐C targets (Figure 2).
Available Tools to Improve Lipid Management Following ACS
Early Systematic Evaluation of the Response to Lipid‐Lowering Therapy
A systematic evaluation of the efficacy of the lipid‐lowering therapy initiated following ACS should be performed early, 4 to 6 weeks after the index event. 20 , 21 Such evaluation is warranted by the significant individual variability in the lipid response to both dietary measures and lipid‐lowering treatment. 57 It also provides the opportunity to monitor the therapeutic adherence and any potential clinical or biological adverse effects. These evaluations may be readily performed during cardiac rehabilitation programs, and may be one of the reasons why these programs have been associated with improved outcomes following ACS. 58 When cardiac rehabilitation programs are not available, nurse‐coordinated care programs may be an interesting alternative, as they have been associated with a significant improvement in the achievement of LDL‐C targets compared with standard‐of‐care follow‐up. 59 An insufficient response to the initiated treatment (LDL‐C reduction <50% from baseline, when not explained by poor therapeutic adherence) should trigger the intensification of lipid‐lowering therapy. 20 , 21
Lipid‐Lowering Intensification: Ezetimibe
Ezetimibe is a potent inhibitor of intestinal absorption of dietary and biliary free cholesterol. 60 In the pivotal IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), the association of ezetimibe on top of maximal dosage of simvastatin led to a significant, albeit limited, reduction of the risk of cardiovascular death, major coronary event, or nonfatal stroke in patients with recent ACS. 25 When looking at specific high‐risk subsets of patients included in the trial, an even larger effect was observed. 61 In fact, there were some significant statistical interactions, suggesting a greater reduction of the primary composite end point with the addition of ezetimibe among patients with diabetes mellitus (hazard ratio, 0.85 [95% CI, 0.78–0.94] versus 0.98 [95% CI, 0.91–1.04] in patients without diabetes mellitus; P value for interaction 0.023) or prior coronary artery bypass graft surgery (for the primary end point, absolute risk reduction: 8.8% [95% CI, 3.1%–14.6%] versus 1.3% [95% CI, 0.0%–2.6%]; P value for interaction at 0.02). 62 , 63 Consistently, the addition of ezetimibe may be of particular interest in patients aged >75 years or presenting with elevated troponin, CRP, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), or reduced glomerular filtration rate. 64 , 65 As a consequence of IMPROVE‐IT, real‐world registries note an increased use of ezetimibe in clinical practice among patients treated for ACS, resulting in improved LDL‐C target achievement. 34 , 66
Lipid‐Lowering Intensification: Bempedoic Acid
Bempedoic acid is an oral prodrug that is only converted to its active thioester in hepatocytes, the only cell to express the relevant acyl coenzyme A synthetase (ie, no conversion in skeletal muscle), and which then inhibits the ATP citrate lyase, a key enzyme of the cholesterol‐biosynthesis pathway. 67 The CLEAR (Cholesterol Lowering via Bempedoic Acid, an adenosinetriphosphate citrate lyase (ACL)‐Inhibiting Regimen) and CLEAR wisdom phase 3 trials recently demonstrated that a regimen of 180 mg once‐a‐day bempedoic acid, in addition to maximally tolerated statin therapy, led to an additional 15% to 20% reduction of LDL‐C plasma levels, with a good safety profile. 68 , 69 The results of these trials were further confirmed by a meta‐analysis of 7 studies comprising 4236 patients. 70 The Food and Drug Administration recently approved bempedoic acid for the treatment of adults with heterozygous familial hypercholesterolemia or established cardiovascular disease and LDL‐C >70 mg/dL despite maximally tolerated statins. Nonetheless, the impact of bempedoic acid on outcomes remains to be determined and is being investigated in the ongoing CLEAR OUTCOMES trial, which recently completed enrollment of 14 014 patients with statin intolerance and high cardiovascular risk or established cardiovascular disease (NCT02993406). Of note, specific data on the impact of bempedoic acid on LDL‐C level reduction and outcomes following a recent ACS are lacking as the dedicated trials have so far excluded patients with a recent ACS.
PCSK9 Inhibitors in Patients With Residual Cholesterol Risk Despite Optimal Lipid‐Lowering Therapy
Nearly 2 decades ago, PCSK9 emerged as a therapeutic target to treat hypercholesterolemia after observational registries reported nonsense mutations of the PCSK9 gene to be associated with a substantial reduction of LDL‐C levels and incidence of coronary events. 71 At the current time, 2 fully human monoclonal antibody PCSK9 inhibitors, alirocumab and evolocumab, are approved by the US Food and Drug Administration. A third agent, the humanized monoclonal antibody bococizumab, was being investigated until the program was halted because of reduced long‐term efficacy attributable to the formation of antidrug antibodies. 72 This phenomenon was not observed with alirocumab or evolocumab. Alirocumab and evolocumab were evaluated in numerous phase 2 and 3 randomized clinical trials of the ODYSSEY and PROFICIO (Program to Reduce LDL‐C and Cardiovascular Outcomes Following Inhibition of PCSK9 in Different Populations) research programs, respectively. 13 The largest randomized controlled trials, the FOURIER and ODYSSEY OUTCOMES, included patients with baseline LDL‐C >70 mg/dL despite optimized lipid‐lowering therapy, and evaluated evolocumab in patients with established cardiovascular disease and alirocumab in patients with recent ACS, respectively. 23 , 24 Both agents were associated with a significant reduction of the primary composite end points, which included death from cardiovascular causes, MI, stroke, and unplanned hospitalization for coronary artery causes. 23 , 24 A recent meta‐analysis centered on these 2 agents, comprising 39 randomized controlled trials, 66 478 patients, and a mean weighted follow‐up time across trials of 2.3 years, reported that PCSK9 inhibition was associated with a significant reduction of MI, ischemic stroke, and coronary revascularization compared with placebo, albeit without a significant reduction of all‐cause and cardiovascular death. 13 , 73 Interestingly, the use of PCSK9 inhibitors was also associated with a reduction of coronary atheroma volume, as measured by intravascular ultrasonography, as well as arterial wall inflammation, as assessed by 18F‐fluoro‐deoxyglucose positron emission tomography/computed tomography, as reported by the GLAGOV (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound) and ODYSSEY J‐IVUS trials, respectively. 74 , 75 , 76 Carotid artery regression with alirocumab was recently reported using magnetic resonance imaging, showing depletion of plaque lipid stores at 6 months. 77 The PACMAN‐AMI (Vascular Effects of Alirocumab in Acute MI‐Patients) trial is currently ongoing (NCT03067844) and will provide a serial and multivessel evaluation of the impact of PCSK9 inhibitors on plaque burden and composition using intravascular ultrasonography, near‐infrared spectroscopy, and optical coherence tomography. Moreover, the HUYGENS (High‐Resolution Assessment of Coronary Plaques in a Global Evolocumab Randomized Study) is evaluating the effect of evolocumab on fibrous cap thickness by optical coherence tomography in patients presenting with non–ST‐segment–elevation MI (NCT03570697). To date, data on the use of PCSK9 inhibitors in patients with recent ACS are mainly limited to alirocumab. Nonetheless, a prespecified analysis from the FOURIER trial, including 5711 patients with a recent MI (<12 months before randomization), showed them to be at a higher risk of adverse events and to experience a greater absolute risk reduction with PCSK9 inhibition compared with patients with a prior MI >12 months before randomization (n=16 609). 78 In the ODYSSEY OUTCOMES trial, use of alirocumab on top of optimized lipid‐lowering therapy was associated with a significant reduction of the risk of stroke, irrespective of baseline LDL‐C and history of cerebrovascular disease, as well as a reduction in the risk of type 2 MI. 79 , 80 More important, in a prespecified subanalysis of patients with at least 3 years of follow‐up available, alirocumab reduced all‐cause death by 22%. 81 , 82 The survival benefit was significantly more pronounced in patients with baseline LDL‐C ≥100 mg/dL, with a 29% relative reduction of mortality (P value for interaction=0.007). 81 The beneficial impact of alirocumab was present in both younger and elderly patients. As the absolute risk of major adverse cardiovascular events increased with age, so did the absolute benefit of alirocumab with a number needed to treat for major adverse cardiovascular events at 3 years at 43 (range, 25–186) patients at age 45 years; 26 (range, 15–97) at age 75 years; and 12 (range, 6–81) at age 85 years. 76 Furthermore, the use of alirocumab was associated with an ≈25% reduction of lipoprotein(a) level in patients after ACS, which is consistent with previous findings on evolocumab in patients with established cardiovascular disease. 83 , 84 Interestingly, in the setting of post‐ACS, the reduction of lipoprotein(a) by PCSK9 inhibitors is independently associated with the reduction of cardiovascular events, leading to a significant reduction of the absolute ischemic risk, particularly in case of high baseline lipoprotein(a). 83 , 84
There are limited data on the initiation of PCSK9 inhibitors before hospital discharge in such patients. The EVOPACS (Evolocumab for Early Reduction of LDL‐Cholesterol Levels in Patients With Acute Coronary Syndromes) trial evaluated the initiation of evolocumab before hospital discharge among patients with ACS with elevated LDL‐C at baseline, defined as ≥70 mg/dL if already on high‐intensity statins or ≥125 mg/dL in the absence of statins. 85 At 8 weeks of follow‐up, 95.7% of the patients in the evolocumab group reached LDL‐C <70 mg/dL versus 37.6% in the placebo group. The VCU‐AlirocRT (Virginia Commonwealth University Alirocumab Response Trial) reported similar results with the use of alirocumab before hospital discharge for non–ST‐segment–elevation MI. 86
Limits of PCSK9 Inhibitors and Recommendations for Targeting the Highest‐Risk Patients
Despite providing a solid reduction in ischemic events, as shown by multiple large‐scale clinical trials, the main barrier to large‐scale prescription of PCSK9 inhibitors in clinical practice has mainly been their substantial cost. At the time of publication, cost‐effectiveness analyses of the pivotal FOURIER and ODYSSEY OUTCOMES trials have consistently shown that the cost of PCSK9 inhibitors was above the threshold of $100 000 to $150 000 per quality‐adjusted life‐year gained. 87 , 88 To improve the cost‐effectiveness of both agents, in addition to significant medication cost reductions (some of which have already occurred after the publication of previously mentioned cost‐effectiveness studies), it is thus necessary to select the patients with the highest baseline risk who would gain the most from further intensification of lipid‐lowering therapy. 89
Several post hoc analyses from the ODYSSEY OUTCOMES and FOURIER trials demonstrated an increased efficacy of PCSK9 inhibitors in various high ischemic risk subsets, such as patients with diabetes mellitus, high baseline LDL‐C level (>100 mg/dL), polyvascular disease, chronic kidney disease, history of multiple coronary events, and persistent residual inflammatory risk (Figure 4). 74 , 96
Figure 4. High‐risk subjects with improved risk reduction and cost‐effectiveness of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors.
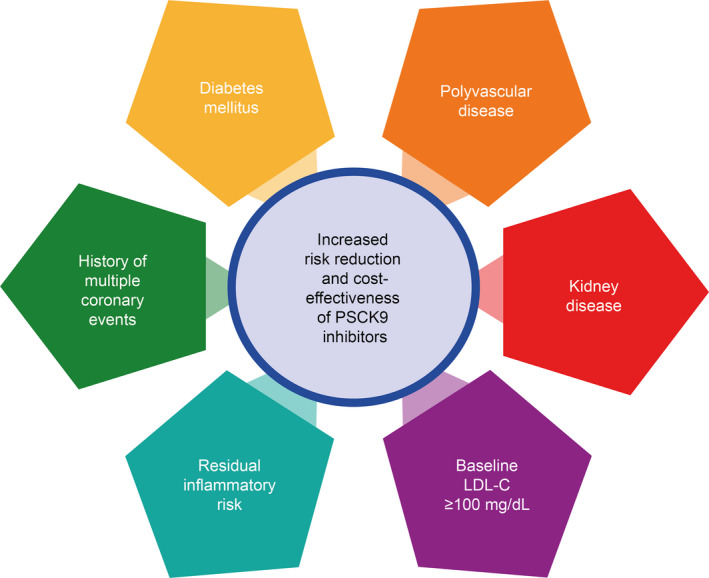
Jukema et al, in an ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) substudy, demonstrated an absolute risk reduction (ARR) of the primary end point with alirocumab of 1.4% (95% CI, 0.6–2.3), 1.9% (95% CI, −2.4% to 6.2%), and 13.0% (95% CI, −2.0% to 28.0%) in patients with single, dual, and triple vascular disease, respectively (P value for interaction=0.0006). Ray et al, in an ODYSSEY OUTCOMES substudy, demonstrated a greater ARR of the primary end point in patients with diabetes mellitus compared with patients with pre–diabetes mellitus or normoglycemia (ARR, 2.3% [95% CI, 0.4%–4.2%], 1.2% [95% CI, 0.0%–2.4%], and 1.2% [95% CI, −0.3% to 2.7%], respectively) (P value for interaction=0.0019). Sabatine et al, from a FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) substudy, found a greater ARR with evolocumab in case of multiple prior myocardial infarctions (3.7% [95% CI, 0.8%–6.6%] vs 1.3% [95% CI, −0.2% to 2.7%]; P value for interaction=0.15). Bohula et al, in a substudy from FOURIER, found a higher ARR of the primary end point in patients with baseline hs‐CRP (high‐sensitivity C‐reactive protein) level >3 mg/L, compared with those with <1 and 1 to 3 mg/L (2.6% [95% CI, 0.4%–4.9%], 1.8% [95% CI, 0.0%–3.5%], and 1.6% [95% CI, −0.5% to 3.7%], respectively). Charytan et al, from a substudy of FOURIER, reported evolocumab to be associated with greater ARR for the key secondary end point. In the ODYSSEY OUTCOMES trial, patients with baseline low‐density lipoprotein cholesterol (LDL‐C) level >100 mg/dL presented with the greatest ARR compared with patients with LDL‐C <80 or 80 to 100 mg/dL (3.4% [95% CI, 1.6%–5.2%] vs 0.3% [95% CI, −1.2% to 1.8%] and 1.3% [95% CI, −0.1% to 2.6%], respectively; P value for interaction <0.001).
Novel Targets and Therapeutic Approaches
Targeting RNA
Another way of lowering PCSK9, aside from using specific blocking antibodies that require administration once or twice a month, is to inhibit gene expression by neutralizing targeted mRNA with small interfering RNA. Inclisiran is a chemically modified double‐stranded small interfering RNA administered subcutaneously with a prolonged effect against PCSK9 synthesis in hepatocytes (Figure 5). 97 , 98 In the recently published ORION‐10 (Inclisiran for Participants With Atherosclerotic Cardiovascular Disease and Elevated Low‐Density Lipoprotein Cholesterol) and ORION‐11 (Inclisiran for Subjects With ACSVD or ACSVD‐Risk Equivalents and Elevated Low‐Density Lipoprotein Cholesterol) phase 3 trials, inclisiran was compared with placebo on top of optimized lipid‐lowering therapy in patients with established ASCVD, or ASCVD risk equivalent, and elevated LDL‐C (≥70 or ≥100 mg/dL, respectively). 99 Compared with placebo, LDL‐C levels were halved with inclisiran, administered on day 1 and day 90, and every 6 months thereafter, without significant difference in terms of safety events aside from injection‐site reactions. The ongoing ORION‐4 (Inclisiran on Clinical Outcomes Among People With Cardiovascular Disease) trial (NCT03705234) will evaluate the impact of inclisiran on ≈15 000 patients with established cardiovascular disease, including a prior MI, although patients with an acute coronary event within 4 weeks of randomization will be excluded. The primary end point will be the composite of death from coronary heart disease, MI, stroke, or urgent coronary revascularization. 100
Figure 5. Mechanism of anti–PCSK9 (proprotein convertase subtilisin/kexin type 9) small interfering RNA (siRNA).

LDL‐C indicates low‐density lipoprotein cholesterol; and RISC, RNA‐induced silencing complex.
Apolipoprotein A1, HDL, and Cholesterol Efflux
Cholesterol efflux, or reverse cholesterol transport, refers to the process by which the excess cholesterol from peripheral (ie, extrahepatic) tissues is returned to the liver for excretion in the bile and feces. 101 It has been demonstrated that cholesterol efflux from arterial macrophages in particular plays an essential role in the prevention of atherosclerosis. 102 In fact, unesterified cholesterol is toxic to macrophages, and overloading may lead to the creation of foam cells, and subsequently their apoptosis or necrosis. Various pathways of cholesterol efflux may be involved, such as efflux to mature HDL‐C via the ATP‐binding cassette transporter G1 or scavenger receptor class B type I, or efflux to lipid‐poor apolipoproteins, such as apolipoprotein A‐I mediated by the ATP‐binding cassette transporter A1. 101 By promoting these pathways, HDL prevents LDL‐induced macrophage apoptosis and endothelial dysfunction. 103 , 104 A growing body of evidence has demonstrated the strong association between HDL‐C efflux capacity and the incidence of major adverse cardiovascular events. 105 For the particular setting of patients undergoing primary percutaneous coronary intervention for acute MI, a recent study demonstrated that reduced serum cholesterol efflux capacity was strongly associated with long‐term mortality, independently of HDL‐C and LDL‐C levels. 106
The AEGIS‐1 (Apo‐I Event Reducing in Ischemic Syndromes I) trial was a phase 2b study evaluating the tolerance of CSL112, a reconstituted injectable human plasma‐derived apolipoprotein A‐I, administered to 1258 patients within 7 days of an acute MI. The trial reported that CSL112 enhanced cholesterol efflux without significant alterations in liver or kidney function. 107 The results of the AEGIS‐1 trial were further confirmed by the CSL112_2001 trial, which included patients presenting with moderate chronic kidney disease. 108 The ongoing AEGIS‐2 trial (NCT03473223) is investigating whether enhancement of cholesterol efflux could result in significant reduction of hard clinical end points. This international, multicenter, randomized clinical trial will include 17 400 patients with recent MI, multivessel CAD, and established cardiovascular risk factors. Such an adequately powered large‐scale trial is of importance, as the use of other synthesized lipid‐poor HDL mimetics, such as CER‐001 or MDCO‐216, was previously not found to be effective in inducing durable regression of coronary atherosclerosis following ACS. 109 , 110
N‐3 Fatty Acids
Observational studies have long reported an association between regular fish consumption and reduction of cardiovascular events. 111 Omega‐3 fatty acids, such as eicosapentaenoic acid (EPA) or docosahexanoic acid, are long‐chain polyunsaturated fatty acids contained in fish oils, with known anti‐inflammatory properties. 112 Nonetheless, numerous large randomized controlled trials and meta‐analyses evaluating such omega‐3 fatty acids, although with lower daily dosage, failed to demonstrate a significant reduction in the rates of cardiovascular events. 113 This recently changed with the REDUCE‐IT (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial), 114 which evaluated a high‐dosage (ie, 4 g/d) treatment with icosapent ethyl, a stable EPA ethyl ester, in 8179 patients with established cardiovascular disease, or with diabetes mellitus and other risk factors, presenting with triglycerides levels of 135 to 499 mg/dL (1.52–5.63 mmol/L) and LDL‐C levels of 40 to 100 mg/dL (1.06–2.59 mmol/L) on stable statin treatment. With a median follow‐up of 4.9 years, the trial reported icosapent ethyl to be associated with a significant (25%) reduction in the primary end point of cardiovascular death, MI, stroke, coronary revascularization, or unstable angina, as well as a significant reduction of the risk of cardiovascular death (20%), sudden cardiac death (31%), and cardiac arrest (48%).The main adverse effects of the use of icosapent ethyl were a slight increase of the risk of atrial fibrillation and peripheral edema. Of importance, 70.7% of the patients included in REDUCE‐IT were treated for secondary prevention, mainly for CAD, such as with documented prior MI or hospitalization for non–ST‐segment–elevation MI, with no predefined minimal delay from the index event. Therefore, a significant proportion treated for an ACS could benefit from icosapent ethyl treatment. In fact, a recent study on a French registry of real‐world patients hospitalized for an MI found that 12.5% of them presented with the inclusion criteria of REDUCE‐IT. 115
The specific mechanisms of action by which daily treatment of a high dosage of icosapent ethyl may lead to such an impressive effect remain to be further clarified, and may represent an effect on plaque progression and stability that may be independent of the triglyceride‐lowering effect. 116 In fact, a significant reduction of the incidence of the primary end point was observed in patients treated with icosapent ethyl whether or not they reached a target triglycerides level <150 mg/dL after 1 year of treatment. The recently published EVAPORATE (Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy) trial evaluated the impact of 4 g/d of icosapent ethyl in 80 patients with elevated triglycerides levels, LDL‐C levels between 40 and 115 mg/dL, and at least 20% stenosis of a coronary artery at angiogram or computed tomography. 117
After 18 months of treatment, treatment with icosapent ethyl was associated with a significant reduction of the volume of low‐attenuation, fibrous, and fatty plaques, which all increased with placebo. Interestingly enough, this effect on plaque volume was observed while no significant difference in terms of LDL‐C or triglycerides levels was present, further emphasizing a potential non–triglyceride‐mediated pleiotropic effect of icosapent ethyl. 118 , 119 , 120 , 121 , 122 Previous studies have reported that EPA treatment could inhibit vascular inflammation via the production of resolvins and protectins, 120 reduce high‐sensitivity CRP levels, and improve vascular function. 122 In contrast, STRENGTH (The Outcomes Study to Assess Statin Residual Risk Reduction With Epanova in High CV Risk Patients With Hypertriglyceridemia) trial, which investigated treatment with Epanova, 4 g once daily, an omega‐3 carboxylic acid, in ≈13 000 patients with an elevated triglyceride level (≥180 mg/dL) and low LDL‐C (<100 mg/dL) who had established atherosclerotic disease or diabetes mellitus and other cardiovascular risk factors (NCT02104817), was recently prematurely halted because of futility. This suggests that the positive results observed with REDUCE‐IT were not solely explained by the use of high dosage of EPA but likely by a unique impact of icosapent ethyl, which was also associated, at the moderate dosage of 1.8 g daily, with a significant 19% reduction of the risk of major coronary events in the JELIS (Japan EPA Lipid Intervention Study). 123
Conclusions
Despite important advances in the initial treatment and secondary prevention of ACS, ≈20% of ACS survivors experience a subsequent ischemic cardiovascular event within 24 months; 5‐year mortality ranges from 19% to 22%. Knowledge of the current state of evidence‐based lipid management after ACS is of paramount importance to improve outcomes after ACS. Guidelines recommend obtaining a lipid profile soon after admission for ACS. A systematic evaluation of the efficacy of the lipid‐lowering therapy initiated following ACS should be performed early, with an LDL‐C reduction target of >50% from baseline. Three agents with well‐documented safety and efficacy can be prescribed: statins, PCSK9 inhibitors, and ezetimibe. PCSK9 inhibitors provide a consistent reduction in ischemic events, as shown by multiple large‐scale clinical trials with lack of major safety concerns. The main barrier to the widespread prescription of these drugs relates to their considerable costs when compared with other lipid‐lowering agents. However, the progressive reduction in manufacturing costs observed with PCSK9 inhibitors may enhance their cost‐effectiveness in daily practice as well as potentially leading to a paradigm shift in the management of high‐risk patients, such as those with an ACS. Eventually, contemporary innovations in lipid‐lowering pharmacotherapies alongside continuous medical education will enable patients to achieve guideline‐directed LDL‐C targets, and will improve outcomes in this vulnerable population.
Sources of Funding
None.
Disclosures
Dr Gibson reports receiving grant support and consulting fees from Angel Medical, Bayer, CSL Behring, Janssen Pharmaceuticals, Johnson & Johnson, and Portola Pharmaceuticals; consulting fees from the Medicines Company, Eli Lilly, Gilead Sciences, Novo Nordisk, WebMD, UpToDate Cardiovascular Medicine, Amarin Pharma, Amgen, Boehringer Ingelheim, Chiesi, Merck, PharmaMar, Sanofi, Somahlution, Verreseon Corporation, Boston Scientific, Impact Bio, MedImmume, Medtelligence, MicroPort, PERT Consortium, and GE Healthcare; holding equity in nference, Inc; serving as chief executive officer of the Baim Institute; and receiving grant support paid to Baim Institute from Bristol‐Myers Squibb. Dr Angiolillo reports grants and personal fees from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Janssen, Merck, Sanofi, and CeloNova; personal fees from Abbott, Haemonetics, PhaseBio, PLx Pharma, Pfizer, The Medicines Company, and St Jude Medical; and grants from CSL Behring, Eisai, Idorsia Pharmaceuticals Ltd, Matsutani Chemical Industry Co, Novartis, Osprey Medical, Renal Guard Solutions, and Scott R. MacKenzie Foundation, outside the submitted work. Dr Lepor reports grants, personal fees, and other from Sanofi, grants and personal fees from Regeneron, personal fees and other from Amgen, outside the submitted work. Dr Mehran reports grants, personal fees, and other funding from Abbott Laboratories; grants from AstraZeneca, Bayer, Beth Israel Deaconess, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Medtronic, Novartis Pharmaceuticals, and Orbus Neich; grants and other funding from Bristol‐Myers Squibb; other funding from Abiomed, The Medicines Company, Spectranetics/Philips/Volcano Corp, Watermark Research Partners, Claret Medical, and Elixir Medical; personal fees from Boston Scientific, Janssen Scientific Affairs, Medscape/WebMD, Roivant Services, Sanofi, Siemens Medical Solutions, Medtelligence (Janssen Scientific Affairs), American College of Cardiology, and American Medical Association; and nonfinancial support and other funding from Idorsia Pharmaceuticals and Regeneron Pharmaceuticals, Inc, outside the submitted work. The remaining authors have no disclosures to report.
Acknowledgments
The authors developed the concept and wrote and approved the publication drafts. Authors received no honoraria related to the development of this publication. Employees of Sanofi and Regeneron Pharmaceuticals, Inc, were permitted to review the manuscript and offer comments. However, the authors were responsible for all content and editorial decisions. Final formatting of references was provided by Michele Damo, PharmD, of Prime, Knutsford, UK, funded by Sanofi and Regeneron Pharmaceuticals, Inc, according to Good Publication Practice guidelines.
(J Am Heart Assoc. 2020;9:e018897. DOI: 10.1161/JAHA.120.018897.)
For Sources of Funding and Disclosures, see page 12.
References
- 1. Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief. 2012:1–8[E-pub ahead of print]. [PubMed] [Google Scholar]
- 3. MacMillan RL, Brown KW. Comparison of the effects of treatment of acute myocardial infarction in a coronary unit and on a general medical ward. Can Med Assoc J. 1971;105:1037–1040. [PMC free article] [PubMed] [Google Scholar]
- 4. Puymirat E, Simon T, Cayla G, Cottin Y, Elbaz M, Coste P, Lemesle G, Motreff P, Popovic B, Khalife K, et al. Acute myocardial infarction: changes in patient characteristics, management, and 6‐month outcomes over a period of 20 years in the FAST‐MI Program (French Registry of Acute ST‐Elevation or Non‐ST‐Elevation Myocardial Infarction) 1995 to 2015. Circulation. 2017;136:1908–1919. [DOI] [PubMed] [Google Scholar]
- 5. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post‐myocardial infarction patients: nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J. 2015;36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 6. Fox KA, Carruthers KF, Dunbar DR, Graham C, Manning JR, De Raedt H, Buysschaert I, Lambrechts D, Van de Werf F. Underestimated and under‐recognized: the late consequences of acute coronary syndrome (GRACE UK‐Belgian Study). Eur Heart J. 2010;31:2755–2764. [DOI] [PubMed] [Google Scholar]
- 7. Palmerini T, Della Riva D, Biondi‐Zoccai G, Leon MB, Serruys PW, Smits PC, von Birgelen C, Ben‐Yehuda O, Généreux P, Bruno AG, et al. Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention: an individual patient data pooled analysis of 21 randomized trials and 32,524 patients. JACC Cardiovasc Interv. 2018;11:892–902. [DOI] [PubMed] [Google Scholar]
- 8. Giustino G, Serruys PW, Sabik JF III, Mehran R, Maehara A, Puskas JD, Simonton CA, Lembo NJ, Kandzari DE, Morice MC, et al. Mortality after repeat revascularization following PCI or CABG for left main disease: the EXCEL trial. JACC Cardiovasc Interv. 2020;13:375–387. [DOI] [PubMed] [Google Scholar]
- 9. Parasca CA, Head SJ, Milojevic M, Mack MJ, Serruys PW, Morice MC, Mohr FW, Feldman TE, Colombo A, Dawkins KD, et al. Incidence, characteristics, predictors, and outcomes of repeat revascularization after percutaneous coronary intervention and coronary artery bypass grafting: the SYNTAX trial at 5 years. JACC Cardiovasc Interv. 2016;9:2493–2507. [DOI] [PubMed] [Google Scholar]
- 10. Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient‐level pooled analysis of the REPLACE‐2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS‐AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654–664. [DOI] [PubMed] [Google Scholar]
- 11. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 12. Cholesterol Treatment Trialists Collaboration , Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guedeney P, Giustino G, Sorrentino S, Claessen BE, Camaj A, Kalkman DN, Vogel B, Sartori S, De Rosa S, Baber U, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta‐analysis of randomized controlled trials. Eur Heart J. 2019:ehz430. [Epub‐ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP. Efficacy and safety of further lowering of low‐density lipoprotein cholesterol in patients starting with very low levels: a meta‐analysis. JAMA Cardiol. 2018;3:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 16. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paciullo F, Momi S, Gresele P. PCSK9 in haemostasis and thrombosis: possible pleiotropic effects of PCSK9 inhibitors in cardiovascular prevention. Thromb Haemost. 2019;119:359–367. [DOI] [PubMed] [Google Scholar]
- 18. Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, Christie JD, Nakada TA, Fjell CD, Thair SA, et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6:258ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoekenbroek RM, Lambert G, Cariou B, Hovingh GK. Inhibiting PCSK9—biology beyond LDL control. Nat Rev Endocrinol. 2018;15:52–62. [DOI] [PubMed] [Google Scholar]
- 20. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 21. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 22. Cartier LJ, Collins C, Lagace M, Douville P. Comparison of fasting and non‐fasting lipid profiles in a large cohort of patients presenting at a community hospital. Clin Biochem. 2018;52:61–66. [DOI] [PubMed] [Google Scholar]
- 23. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 25. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 26. Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, Krumholz HM. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–2797. [DOI] [PubMed] [Google Scholar]
- 27. Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen SJ, Chen TJ, Lin SJ, Chiang CE. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231–1238. [DOI] [PubMed] [Google Scholar]
- 28. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, et al. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA. 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 29. Waters DD, Ho JE, DeMicco DA, Breazna A, Arsenault BJ, Wun CC, Kastelein JJ, Colhoun H, Barter P. Predictors of new‐onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol. 2011;57:1535–1545. [DOI] [PubMed] [Google Scholar]
- 30. Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, Xie J, Kang LN, Xu B. Safety and efficacy of anti‐PCSK9 antibodies: a meta‐analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633–643. [DOI] [PubMed] [Google Scholar]
- 32. Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, Trikalinos TA. Do statins impair cognition? A systematic review and meta‐analysis of randomized controlled trials. J Gen Intern Med. 2015;30:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipinski MJ, Benedetto U, Escarcega RO, Biondi‐Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R. The impact of proprotein convertase subtilisin‐kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta‐analysis. Eur Heart J. 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 34. Gencer B, Carballo D, Nanchen D, Koskinas KC, Klingenberg R, Raber L, Auer R, Carballo S, Heg D, Windecker S, et al. Intensified lipid lowering using ezetimibe after publication of the IMPROVE‐IT trial: a contemporary analysis from the SPUM‐ACS cohort. Int J Cardiol. 2020;303:8–13. [DOI] [PubMed] [Google Scholar]
- 35. Colantonio LD, Rosenson RS, Deng L, Monda KL, Dai Y, Farkouh ME, Safford MM, Philip K, Mues KE, Muntner P. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc. 2019;8:e010376. DOI: 10.1161/JAHA.118.010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bittner V, Colantonio LD, Dai Y, Woodward M, Mefford MT, Rosenson RS, Muntner P, Monda KL, Kilgore ML, Jaeger BC, et al. Association of region and hospital and patient characteristics with use of high‐intensity statins after myocardial infarction among Medicare beneficiaries. JAMA Cardiol. 2019;4:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guedeney P, Claessen BE, Baber U, Camaj A, Sorrentino S, Aquino M, Blum M, Chandiramani R, Goel R, Elsayed S, et al. Temporal trends in statin prescriptions and residual cholesterol risk in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Am J Cardiol. 2019;123:1788–1795. [DOI] [PubMed] [Google Scholar]
- 38. Guedeney P, Baber U, Claessen B, Aquino M, Camaj A, Sorrentino S, Vogel B, Farhan S, Faggioni M, Chandrasekhar J, et al. Temporal trends, determinants, and impact of high‐intensity statin prescriptions after percutaneous coronary intervention: results from a large single‐center prospective registry. Am Heart J. 2019;207:10–18. [DOI] [PubMed] [Google Scholar]
- 39. Peters SAE, Colantonio LD, Zhao H, Bittner V, Dai Y, Farkouh ME, Monda KL, Safford MM, Muntner P, Woodward M. Sex differences in high‐intensity statin use following myocardial infarction in the United States. J Am Coll Cardiol. 2018;71:1729–1737. [DOI] [PubMed] [Google Scholar]
- 40. Lauffenburger JC, Robinson JG, Oramasionwu C, Fang G. Racial/ethnic and gender gaps in the use of and adherence to evidence‐based preventive therapies among elderly Medicare Part D beneficiaries after acute myocardial infarction. Circulation. 2014;129:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aarnio E, Martikainen J, Winn AN, Huupponen R, Vahtera J, Korhonen MJ. Socioeconomic inequalities in statin adherence under universal coverage: does sex matter? Circ Cardiovasc Qual Outcomes. 2016;9:704–713. [DOI] [PubMed] [Google Scholar]
- 42. Kolandaivelu K, Leiden BB, O'Gara PT, Bhatt DL. Non‐adherence to cardiovascular medications. Eur Heart J. 2014;35:3267–3276. [DOI] [PubMed] [Google Scholar]
- 43. Zhang H, Plutzky J, Turchin A. Discontinuation of statins in routine care settings. Ann Intern Med. 2013;159:75–76. [DOI] [PubMed] [Google Scholar]
- 44. Pittman DG, Chen W, Bowlin SJ, Foody JM. Adherence to statins, subsequent healthcare costs, and cardiovascular hospitalizations. Am J Cardiol. 2011;107:1662–1666. [DOI] [PubMed] [Google Scholar]
- 45. Ferdinand KC, Senatore FF, Clayton‐Jeter H, Cryer DR, Lewin JC, Nasser SA, Fiuzat M, Califf RM. Improving medication adherence in cardiometabolic disease: practical and regulatory implications. J Am Coll Cardiol. 2017;69:437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, Virani SS, Louie MJ, Lee LV, Peterson ED, et al. Patient‐reported reasons for declining or discontinuing statin therapy: insights from the PALM Registry. J Am Heart Assoc. 2019;8:e011765. DOI: 10.1161/JAHA.118.011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- 48. Ngo‐Metzger Q, Zuvekas S, Shafer P, Tracer H, Borsky AE, Bierman AS. Statin use in the U.S. for secondary prevention of cardiovascular disease remains suboptimal. J Am Board Fam Med. 2019;32:807–817. [DOI] [PubMed] [Google Scholar]
- 49. Yancy CW, Wang TY, Ventura HO, Pina IL, Vijayaraghavan K, Ferdinand KC, Hall LL. The coalition to reduce racial and ethnic disparities in cardiovascular disease outcomes (credo): why credo matters to cardiologists. J Am Coll Cardiol. 2011;57:245–252. [DOI] [PubMed] [Google Scholar]
- 50. Pu J, Hastings KG, Boothroyd D, Jose PO, Chung S, Shah JB, Cullen MR, Palaniappan LP, Rehkopf DH. Geographic variations in cardiovascular disease mortality among Asian American subgroups, 2003–2011. J Am Heart Assoc. 2017;6:e005597. DOI: 10.1161/JAHA.117.005597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferrari R, Ford I, Greenlaw N, Tardif JC, Tendera M, Abergel H, Fox K, Hu D, Shalnova S, Steg PG. Geographical variations in the prevalence and management of cardiovascular risk factors in outpatients with CAD: data from the contemporary CLARIFY registry. Eur J Prev Cardiol. 2015;22:1056–1065. [DOI] [PubMed] [Google Scholar]
- 52. Watanabe JH, Kazerooni R, Bounthavong M. Association of copayment with likelihood and level of adherence in new users of statins: a retrospective cohort study. J Manag Care Pharm. 2014;20:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hlatky MA, Kazi DS. PCSK9 inhibitors: economics and policy. J Am Coll Cardiol. 2017;70:2677–2687. [DOI] [PubMed] [Google Scholar]
- 54. Tanner RM, Safford MM, Monda KL, Taylor B, O'Beirne R, Morris M, Colantonio LD, Dent R, Muntner P, Rosenson RS. Primary care physician perspectives on barriers to statin treatment. Cardiovasc Drugs Ther. 2017;31:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gurgoze MT, Muller‐Hansma AHG, Schreuder MM, Galema‐Boers AMH, Boersma E, Roeters van Lennep JE. Adverse events associated with PCSK9 inhibitors: a real‐world experience. Clin Pharmacol Ther. 2019;105:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA; The Cochrane Effective Practice and Organization of Care Review Group . Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. BMJ. 1998;317:465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta‐analysis of statin trials. J Am Coll Cardiol. 2014;64:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kureshi F, Kennedy KF, Jones PG, Thomas RJ, Arnold SV, Sharma P, Fendler T, Buchanan DM, Qintar M, Ho PM, et al. Association between cardiac rehabilitation participation and health status outcomes after acute myocardial infarction. JAMA Cardiol. 2016;1:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huber D, Henriksson R, Jakobsson S, Mooe T. Nurse‐led telephone‐based follow‐up of secondary prevention after acute coronary syndrome: one‐year results from the randomized controlled NAILED‐ACS trial. PLoS One. 2017;12:e0183963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. [DOI] [PubMed] [Google Scholar]
- 61. Bohula EA, Morrow DA, Giugliano RP, Blazing MA, He P, Park JG, Murphy SA, White JA, Kesaniemi YA, Pedersen TR, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69:911–921. [DOI] [PubMed] [Google Scholar]
- 62. Eisen A, Cannon CP, Blazing MA, Bohula EA, Park JG, Murphy SA, White JA, Giugliano RP, Braunwald E. The benefit of adding ezetimibe to statin therapy in patients with prior coronary artery bypass graft surgery and acute coronary syndrome in the IMPROVE‐IT trial. Eur Heart J. 2016;37:3576–3584. [DOI] [PubMed] [Google Scholar]
- 63. Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalan R, Spinar J, Park JG, White JA, Bohula EA, Braunwald E, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results from IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2018;137:1571–1582. [DOI] [PubMed] [Google Scholar]
- 64. Bach RG, Cannon CP, Giugliano RP, White JA, Lokhnygina Y, Bohula EA, Califf RM, Braunwald E, Blazing MA. Effect of simvastatin‐ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2019;4:846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qamar A, Giugliano RP, Bohula EA, Park JG, Jarolim P, Murphy SA, Blazing MA, Califf RM, Cannon CP, Braunwald E, et al. Biomarkers and clinical cardiovascular outcomes with ezetimibe in the IMPROVE‐IT Trial. J Am Coll Cardiol. 2019;74:1057–1068. [DOI] [PubMed] [Google Scholar]
- 66. Guglielmi V, Bellia A, Pecchioli S, Della‐Morte D, Parretti D, Cricelli I, Medea G, Sbraccia P, Lauro D, Cricelli C, et al. Effectiveness of adherence to lipid lowering therapy on LDL‐cholesterol in patients with very high cardiovascular risk: a real‐world evidence study in primary care. Atherosclerosis. 2017;263:36–41. [DOI] [PubMed] [Google Scholar]
- 67. Pinkosky SL, Newton RS, Day EA, Ford RJ, Lhotak S, Austin RC, Birch CM, Smith BK, Filippov S, Groot PHE, et al. Liver‐specific ATP‐citrate lyase inhibition by bempedoic acid decreases LDL‐C and attenuates atherosclerosis. Nat Commun. 2016;7:13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL, Ballantyne CM. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 69. Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, Lalwani ND, Patel PM, Zhao X, Duell PB. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low‐density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA. 2019;322:1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Di Minno A, Lupoli R, Calcaterra I, Poggio P, Forte F, Spadarella G, Ambrosino P, Iannuzzo G, Di Minno MND. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia: systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2020;9:e016262. DOI: 10.1161/JAHA.119.016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 72. Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, Kastelein JJ, Kim AM, Koenig W, Nissen S, et al. Lipid‐reduction variability and antidrug‐antibody formation with bococizumab. N Engl J Med. 2017;376:1517–1526. [DOI] [PubMed] [Google Scholar]
- 73. Wiviott SD, Giugliano RP, Morrow DA, De Ferrari GM, Lewis BS, Huber K, Kuder JF, Murphy SA, Forni DM, Kurtz CE, et al. Effect of evolocumab on type and size of subsequent myocardial infarction: a prespecified analysis of the FOURIER randomized clinical trial. JAMA Cardiol. 2020;5:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, et al. Effect of evolocumab on progression of coronary disease in statin‐treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 75. Ako J, Hibi K, Tsujita K, Hiro T, Morino Y, Kozuma K, Shinke T, Otake H, Uno K, Louie MJ, et al. Effect of alirocumab on coronary atheroma volume in japanese patients with acute coronary syndrome—the ODYSSEY J‐IVUS Trial. Circ J. 2019;83:2025–2033. [DOI] [PubMed] [Google Scholar]
- 76. Hoogeveen RM, Opstal TSJ, Kaiser Y, Stiekema LCA, Kroon J, Knol RJJ, Bax WA, Verberne HJ, Cornel JH, Stroes ESG. PCSK9 antibody alirocumab attenuates arterial wall inflammation without changes in circulating inflammatory markers. JACC Cardiovasc Imaging. 2019;12:2571–2573. [DOI] [PubMed] [Google Scholar]
- 77. Kerwin WS, Hatsukami T, Yuan C, Zhao XQ. MRI of carotid atherosclerosis. AJR Am J Roentgenol. 2013;200:W304–W313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gencer B, Mach F, Murphy SA, De Ferrari GM, Huber K, Lewis BS, Ferreira J, Kurtz CE, Wang H, Honarpour N, et al. Efficacy of evolocumab on cardiovascular outcomes in patients with recent myocardial infarction: a prespecified secondary analysis from the FOURIER Trial. JAMA Cardiol. 2020;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jukema JW, Zijlstra LE, Bhatt DL, Bittner VA, Diaz R, Drexel H, Goodman SG, Kim YU, Pordy R, Reiner Z, et al. Effect of alirocumab on stroke in ODYSSEY OUTCOMES. Circulation. 2019;140:2054–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sinnaeve PR, Schwartz GG, Wojdyla DM, Alings M, Bhatt DL, Bittner VA, Chiang CE, Correa Flores RM, Diaz R, Dorobantu M, et al. Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: an ODYSSEY OUTCOMES trial analysis. Eur Heart J. 2020;5:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Steg PG, Szarek M, Bhatt DL, Bittner VA, Bregeault MF, Dalby AJ, Diaz R, Edelberg JM, Goodman SG, Hanotin C, et al. Effect of alirocumab on mortality after acute coronary syndromes. Circulation. 2019;140:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bays HE. Alirocumab, decreased mortality, nominal significance, p values, Bayesian statistics, and the duplicity of multiplicity. Circulation. 2019;140:113–116. [DOI] [PubMed] [Google Scholar]
- 83. Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–144. [DOI] [PubMed] [Google Scholar]
- 84. Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Blom D, Seidah NG, Honarpour N, Lira A, Xue A, Chiruvolu P, et al. PCSK9 inhibition‐mediated reduction in Lp(a) with evolocumab: an analysis of 10 clinical trials and the LDL receptor's role. J Lipid Res. 2016;57:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Koskinas KC, Windecker S, Pedrazzini G, Mueller C, Cook S, Matter CM, Muller O, Haner J, Gencer B, Crljenica C, et al. Evolocumab for early reduction of LDL‐cholesterol levels in patients with acute coronary syndromes (EVOPACS). J Am Coll Cardiol. 2019;74:2452–2462. [DOI] [PubMed] [Google Scholar]
- 86. Trankle CR, Wohlford G, Buckley LF, Kadariya D, Ravindra K, Markley R, Park TS, Potere N, Van Tassell BW, Abbate A. Alirocumab in acute myocardial infarction: results from the Virginia Commonwealth University Alirocumab Response Trial (VCU‐AlirocRT). J Cardiovasc Pharmacol. 2019;74:266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fonarow GC, Keech AC, Pedersen TR, Giugliano RP, Sever PS, Lindgren P, van Hout B, Villa G, Qian Y, Somaratne R, et al. Cost‐effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kazi DS, Penko J, Coxson PG, Guzman D, Wei PC, Bibbins‐Domingo K. Cost‐effectiveness of alirocumab: a just‐in‐time analysis based on the ODYSSEY Outcomes Trial. Ann Intern Med. 2019;170:221–229. [DOI] [PubMed] [Google Scholar]
- 89. Robinson JG, Jayanna MB, Brown AS, Aspry K, Orringer C, Gill EA, Goldberg A, Jones LK, Maki K, Dixon DL, et al. Enhancing the value of PCSK9 monoclonal antibodies by identifying patients most likely to benefit: a consensus statement from the National Lipid Association. J Clin Lipidol. 2019;13:525–537. [DOI] [PubMed] [Google Scholar]
- 90. Jukema JW, Szarek M, Zijlstra LE, de Silva HA, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, et al. Alirocumab in patients with polyvascular disease and recent acute coronary syndrome: ODYSSEY OUTCOMES Trial. J Am Coll Cardiol. 2019;74:1167–1176. [DOI] [PubMed] [Google Scholar]
- 91. Ray KK, Colhoun HM, Szarek M, Baccara‐Dinet M, Bhatt DL, Bittner VA, Budaj AJ, Diaz R, Goodman SG, Hanotin C, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:618–628. [DOI] [PubMed] [Google Scholar]
- 92. Sabatine MS, De Ferrari GM, Giugliano RP, Huber K, Lewis BS, Ferreira J, Kuder JF, Murphy SA, Wiviott SD, Kurtz CE, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease. Circulation. 2018;138:756–766. [DOI] [PubMed] [Google Scholar]
- 93. Charytan DM, Sabatine MS, Pedersen TR, Im K, Park J‐G, Pineda AL, Wasserman SM, Deedwania P, Olsson AG, Sever PS, et al. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER Trial. J Am Coll Cardiol. 2019;73:2961–2970. [DOI] [PubMed] [Google Scholar]
- 94. Bohula EA, Giugliano RP, Leiter LA, Verma S, Park JG, Sever PS, Lira Pineda A, Honarpour N, Wang H, Murphy SA, et al. Inflammatory and cholesterol risk in the FOURIER Trial. Circulation. 2018;138:131–140. [DOI] [PubMed] [Google Scholar]
- 95. Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, et al. Low‐density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137:338–350. [DOI] [PubMed] [Google Scholar]
- 96. Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, Murphy SA, Kuder JF, Gouni‐Berthold I, Lewis BS, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941–950. [DOI] [PubMed] [Google Scholar]
- 97. Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, Wijngaard P, Horton JD, Taubel J, Brooks A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430–1440. [DOI] [PubMed] [Google Scholar]
- 99. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–1519. [DOI] [PubMed] [Google Scholar]
- 100. Stoekenbroek RM, Kallend D, Wijngaard PL, Kastelein JJ. Inclisiran for the treatment of cardiovascular disease: the ORION clinical development program. Future Cardiol. 2018;14:433–442. [DOI] [PubMed] [Google Scholar]
- 101. Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. [DOI] [PubMed] [Google Scholar]
- 102. Khera R, Vaughan‐Sarrazin M, Rosenthal GE, Girotra S. Racial disparities in outcomes after cardiac surgery: the role of hospital quality. Curr Cardiol Rep. 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Terasaka N, Wang N, Yvan‐Charvet L, Tall AR. High‐density lipoprotein protects macrophages from oxidized low‐density lipoprotein‐induced apoptosis by promoting efflux of 7‐ketocholesterol via ABCG1. Proc Natl Acad Sci USA. 2007;104:15093–15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Terasaka N, Yu S, Yvan‐Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high‐cholesterol diet. J Clin Invest. 2008;118:3701–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Guerin M, Silvain J, Gall J, Darabi M, Berthet M, Frisdal E, Hauguel‐Moreau M, Zeitouni M, Kerneis M, Lattuca B, et al. Association of serum cholesterol efflux capacity with mortality in patients with ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2018;72:3259–3269. [DOI] [PubMed] [Google Scholar]
- 107. Michael Gibson C, Korjian S, Tricoci P, Daaboul Y, Yee M, Jain P, Alexander JH, Steg PG, Lincoff AM, Kastelein JJ, et al. Safety and tolerability of CSL112, a reconstituted, infusible, plasma‐derived apolipoprotein A‐I, after acute myocardial infarction: the AEGIS‐I Trial (ApoA‐I Event Reducing in Ischemic Syndromes I). Circulation. 2016;134:1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gibson CM, Kerneis M, Yee MK, Daaboul Y, Korjian S, Mehr AP, Tricoci P, Alexander JH, Kastelein JJP, Mehran R, et al. The CSL112‐2001 trial: safety and tolerability of multiple doses of CSL112 (apolipoprotein A‐I [human]), an intravenous formulation of plasma‐derived apolipoprotein A‐I, among subjects with moderate renal impairment after acute myocardial infarction. Am Heart J. 2019;208:81–90. [DOI] [PubMed] [Google Scholar]
- 109. Nicholls SJ, Puri R, Ballantyne CM, Jukema JW, Kastelein JJP, Koenig W, Wright RS, Kallend D, Wijngaard P, Borgman M, et al. Effect of infusion of high‐density lipoprotein mimetic containing recombinant apolipoprotein A‐I Milano on coronary disease in patients with an acute coronary syndrome in the MILANO‐PILOT trial: a randomized clinical trial. JAMA Cardiol. 2018;3:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nicholls SJ, Andrews J, Kastelein JJP, Merkely B, Nissen SE, Ray KK, Schwartz GG, Worthley SG, Keyserling C, Dasseux JL, et al. Effect of serial infusions of CER‐001, a pre‐beta high‐density lipoprotein mimetic, on coronary atherosclerosis in patients following acute coronary syndromes in the CER‐001 atherosclerosis regression acute coronary syndrome trial: a randomized clinical trial. JAMA Cardiol. 2018;3:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB. Fish consumption and the 30‐year risk of fatal myocardial infarction. N Engl J Med. 1997;336:1046–1053. [DOI] [PubMed] [Google Scholar]
- 112. Zhang MJ, Spite M. Resolvins: anti‐inflammatory and proresolving mediators derived from omega‐3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–227. [DOI] [PubMed] [Google Scholar]
- 113. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, et al. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 115. Ferrières J, Bataille V, Puymirat E, Schiele F, Simon T, Danchin N. Applicability of the REDUCE‐IT trial to the FAST‐MI registry: are the results of randomized trials relevant in routine clinical practice? Clin Cardiol. 2020. [Epub‐ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kastelein JJP, Stroes ESG. FISHing for the miracle of eicosapentaenoic acid. N Engl J Med. 2019;380:89–90. [DOI] [PubMed] [Google Scholar]
- 117. Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200–499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clin Cardiol. 2018;41:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242:357–366. [DOI] [PubMed] [Google Scholar]
- 119. Nelson JR, True WS, Le V, Mason RP. Can pleiotropic effects of eicosapentaenoic acid (EPA) impact residual cardiovascular risk? Postgrad Med. 2017;129:822–827. [DOI] [PubMed] [Google Scholar]
- 120. Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega‐3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21:781–792. [DOI] [PubMed] [Google Scholar]
- 121. Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013;13:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dangardt F, Osika W, Chen Y, Nilsson U, Gan LM, Gronowitz E, Strandvik B, Friberg P. Omega‐3 fatty acid supplementation improves vascular function and reduces inflammation in obese adolescents. Atherosclerosis. 2010;212:580–585. [DOI] [PubMed] [Google Scholar]
- 123. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]


