Abstract
Background
Ventilation with the noble gas argon (Ar) has shown neuroprotective and cardioprotective properties in different in vitro and in vivo models. Hence, the neuroprotective effects of Ar were investigated in a severe, preclinically relevant porcine model of cardiac arrest.
Methods and Results
Cardiac arrest was ischemically induced in 36 pigs and left untreated for 12 minutes before starting cardiopulmonary resuscitation. Animals were randomized to 4‐hour post‐resuscitation ventilation with: 70% nitrogen–30% oxygen (control); 50% Ar–20% nitrogen–30% oxygen (Ar 50%); and 70% Ar–30% oxygen (Ar 70%). Hemodynamic parameters and myocardial function were monitored and serial blood samples taken. Pigs were observed up to 96 hours for survival and neurological recovery. Heart and brain were harvested for histopathology. Ten animals in each group were successfully resuscitated. Ninety‐six‐hour survival was 60%, 70%, and 90%, for the control, Ar 50%, and Ar 70% groups, respectively. In the Ar 50% and Ar 70% groups, 60% and 80%, respectively, achieved good neurological recovery, in contrast to only 30% in the control group (P<0.0001). Histology showed less neuronal degeneration in the cortex (P<0.05) but not in the hippocampus, and less reactive microglia activation in the hippocampus (P=0.007), after Ar compared with control treatment. A lower increase in circulating biomarkers of brain injury, together with less kynurenine pathway activation (P<0.05), were present in Ar‐treated animals compared with controls. Ar 70% pigs also had complete left ventricular function recovery and smaller infarct and cardiac troponin release (P<0.01).
Conclusions
Post‐resuscitation ventilation with Ar significantly improves neurologic recovery and ameliorates brain injury after cardiac arrest with long no‐flow duration. Benefits are greater after Ar 70% than Ar 50%.
Keywords: argon, cardiac arrest, neurological outcome, noble gas, treatment
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- Ar
argon
- CA
cardiac arrest
- ERK
extracellular signal‑regulated kinase
- EtCO2
end‐tidal partial pressure of carbon dioxide
- hs‐cTnT
high‐sensitivity cardiac troponin T
- Iba1
ionized calcium‐binding adaptor molecule 1
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MPTP
mitochondrial permeability transition pore
- VF
ventricular fibrillation
Clinical Perspective
What Is New?
Ventilation with argon significantly improves neurologic recovery after cardiac arrest with a long no‐flow duration.
Argon‐mediated neuroprotection is more pronounced at high concentration, ie, 70%.
Ventilation with argon is safe with no detrimental effects on hemodynamics and respiratory gas exchanges.
What Are the Clinical Implications?
The study introduces a treatment protocol for neuroprotection ready to be clinically translated.
The study results support 4‐hour ventilation with a mixture of argon 70%–oxygen 30% initiated immediately after return of spontaneous circulation.
Survival after successful resuscitation from cardiac arrest (CA) is disappointingly low, with only ≈10% of patients discharged alive from the hospital and with wide differences among countries. 1 , 2 , 3 The majority of resuscitated patients die in the hospital from "post‐CA syndrome," a pathophysiological condition involving multiple organs and mainly characterized by myocardial failure and ischemic brain damage. 4 , 5 Even though post‐CA care has evolved over recent decades, brain injury accounts for the greater part of late deaths in the hospital and patients who eventually survive frequently experience persistent neurocognitive impairment that heavily affects their quality of life. 1 , 5 , 6 , 7
A number of novel therapeutic approaches have been conceived for cerebral preservation after CA. In addition to the still controversial therapeutic hypothermia, 8 , 9 ventilation with noble gases is probably the most attractive clinical option among the interventions proposed. 10 The noble gas argon (Ar) has recently shown neuroprotective and cardioprotective properties in different in vitro and in vivo models of hypoxic‐ischemic insult. 11 Encouraging data have been reported in animal studies on CA in which better and faster neurological recovery was seen, together with less tissue injury, when ventilation with Ar was delivered during the post‐resuscitation period. 12 , 13 , 14
In a porcine model of 8 minutes of untreated CA, we previously demonstrated that post‐resuscitation ventilation with an inhaled mixture of 70% Ar–30% oxygen achieved complete neurological recovery 24 hours after the return of spontaneous circulation, in contrast to control ventilation with 70% nitrogen–30% oxygen. 14 However, the short interval of untreated CA in that study resulted only in a mild brain injury, not reproducing the outcomes observed in the clinical scenario, and represented a clear limitation. The extent of neuronal damage and impact on outcome is known to be contingent on the duration of ischemia. 15 Thus, further studies confirming the protection played by Ar in more severe ischemia models were needed to pave the way toward its clinical application. 14
The aim of this study was to investigate whether ventilation with Ar is neuroprotective even after more severe CA with long no‐flow duration in a preclinically relevant porcine model. 14 , 15 , 16 Two different inhaled Ar concentrations were compared in order to identify the dose with the greatest protective effect. We hypothesized that Ar‐induced neuroprotection would persist after severe prolonged CA and that the effects would be more pronounced for ventilation at higher Ar concentrations.
Methods
All procedures involving animals and their care conformed with national and international laws and policies. Approval of the study was obtained from the institutional review board and governmental institution (Ministry of Health approval no. 84/2014‐PR). The data that support the findings of this study are available from the corresponding author on reasonable request.
Animal Preparation
Thirty‐six male domestic pigs (39±2 kg) were fasted the night before the experiment, with free access to water. Anesthesia was induced by intramuscular injection of ketamine (20 mg/kg) followed by intravenous propofol (2 mg/kg) and sufentanil (0.3 μg/kg) through an ear vein access. Anesthesia was then maintained by continuous intravenous infusion of propofol (4–8 mg/kg per hour) and sufentanil (0.3 μg/kg per hour). A cuffed tracheal tube was placed, and animals were mechanically ventilated with a tidal volume of 15 mL/kg and fraction of inspired oxygen 0.21. Respiratory frequency was adjusted to maintain the end‐tidal partial pressure of carbon dioxide (EtCO2) between 35 and 40 mm Hg, monitored with an infrared capnometer. 14 , 15 , 16 To measure aortic pressure, a fluid‐filled 7F catheter was advanced from the right femoral artery into the thoracic aorta. To measure right atrial pressure, core temperature, and cardiac output, a 7F pentalumen thermodilution catheter was advanced from the right femoral vein into the pulmonary artery. Conventional pressure transducers were used (MedexTransStar, Monsey, NY).
Myocardial infarction was induced in a closed‐chest preparation by intraluminal occlusion of the left anterior descending coronary artery. A 6F balloon‐tipped catheter was inserted from the right common carotid artery and advanced into the aorta then into the left anterior descending beyond the first diagonal branch with the aid of image intensification, and confirmed by injection of radiographic contrast media. To induce ventricular fibrillation (VF), a 5F pacing catheter was advanced from the right subclavian vein into the right ventricle. 14 , 15 , 16 The positions of all catheters were confirmed by characteristic pressure morphology and/or fluoroscopy. A frontal plane ECG was recorded.
Experimental Procedures
Fifteen minutes before inducing CA, animals were randomized to receive immediately after resuscitation 4‐hour ventilation with one of the following inhalatory mixtures: (1) 70% nitrogen–30% oxygen (control) 12 ; (2) 50% Ar–20% nitrogen–30% oxygen (Ar 50%) 12 ; and (3) 70% Ar–30% oxygen (Ar 70%). 12 The experimental design is illustrated in Figure 1. Ventilation was provided with a dedicated clinical ventilator (Bellavista 1000, IMT Medical) specifically modified to deliver the different Ar mixtures, supplemented through certified standard medical gas tanks (SIAD).
Figure 1. Experimental design.
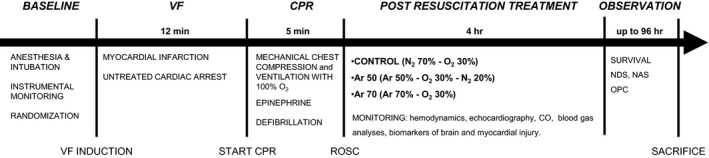
Ar indicates argon; CO, cardiac output; CPR, cardiopulmonary resuscitation; N2, nitrogen; NAS, neurological alertness score; NDS, neurological deficit score; O2, oxygen; OPC, overall performance category; ROSC, return of spontaneous circulation; and VF, ventricular fibrillation.
The left anterior descending coronary artery catheter balloon was then inflated with 0.7 mL of air to occlude the flow, as previously described. 14 , 15 , 16 Occlusion was confirmed by the rapid occurrence of progressive ECG ST‐segment elevation. If VF did not occur spontaneously, after 10 minutes it was induced with 1 to 2 mA AC current delivered to the right ventricular endocardium. Ventilation was discontinued after the onset of VF. After 12 minutes of untreated VF, cardiopulmonary resuscitation (CPR) was started including chest compression with the LUCAS 2 (Physio‐Control Inc) and mechanical ventilation with oxygen (tidal volume 500 mL, 10 breaths per minute; Bellavista 1000, IMT). After 5 minutes of CPR, defibrillation was attempted with a single biphasic 150‐J shock, using an MRx defibrillator (Philips Medical Systems). If resuscitation was not achieved, CPR was resumed and continued for 1 minute before subsequent defibrillation. Adrenaline (30 µg/kg) was administered via the right atrium after 2 and 7 minutes of CPR. Successful resuscitation was defined as restoration of an organized cardiac rhythm with mean arterial pressure >60 mm Hg. After that, if VF reoccurred, it was treated by immediate defibrillation. 14 , 15 , 16 After successful resuscitation, anesthesia was maintained, and animals were monitored during the 4 hours of treatment. Forty‐five minutes after resuscitation, the left anterior descending coronary artery catheter was withdrawn. The animals’ temperature was maintained at 38±0.5°C throughout the whole experiment.
After 4 hours of treatment, catheters were removed, wounds were repaired, and the animals were extubated and returned to their cages. Analgesia with butorphanol (0.1 mg/kg) was given by intramuscular injection. At the end of the 96‐hour post‐resuscitation observation period, animals were re‐anesthetized for echocardiographic examination and blood was sampled. The pigs were then euthanized painlessly with an intravenous injection of 150 mg/kg sodium thiopental, and the heart and brain were harvested. Autopsy was performed routinely to check for any injuries caused by CPR or obfuscating disease.
Measurements
Hemodynamics, EtCO2, and ECG were recorded continuously on a personal computer–based acquisition system (WinDaq DATAQ Instruments Inc). The coronary perfusion pressure was computed from the differences in time‐coincident diastolic aortic pressure and right atrial pressure. Cardiac output was measured by thermodilution (COM‐2, Baxter International Inc). Transthoracic echocardiography was performed with a phase‐array multifrequency 2.5‐ to 5‐MHz probe (CX50, Philips). Two‐dimensional apical 4‐chamber view was acquired to determine left ventricular (LV) volumes and ejection fraction; calculations were performed using the modified single‐plane Simpson rule. 14 , 15 , 16
Arterial blood gases were assessed with the i‐STAT System (Abbott Laboratories) at baseline and 2 and 4 hours post‐resuscitation. Plasma high‐sensitivity cardiac troponin T (hs‐cTnT) at baseline and 2, 4, and 96 hours post‐resuscitation, and serum neuron‐specific enolase at baseline and 96 hours post‐resuscitation, were measured with electrochemiluminescence assays (Roche Diagnostics Italia). Glial fibrillary acidic protein and ubiquitin C‐terminal hydrolase L1 were measured with Simoa Human Neurology 4‐Plex B assay (N4PB) on a Quanterix SIMOA HD‐1 platform, at baseline and 96 hours post‐resuscitation. Plasma levels of tryptophan and its metabolites, kynurenine, kynurenic acid, and 3‐hydroxyanthranilic acid, were measured using high‐performance liquid chromatography coupled with mass spectrometry, adopting the analytical method previously used for human samples 17 (Data S1).
As previously described, 14 , 15 , 16 neurologic recovery 24, 48, and 96 hours post‐resuscitation was assessed with the neurologic alertness score, from 100 (normal) to 0 (brain death), and with the swine neurologic deficit score, ranging from 0 (normal) to 400 (brain death). Finally, functional recovery was evaluated before euthanasia according to the overall performance category as follows: 1, normal; 2, slight disability; 3, severe disability; 4, coma; and 5, brain death or death. Outcome was defined as poor when overall performance category was ≥3. Scores were assessed by veterinarians blinded to treatments.
The brains were carefully removed from the skull and fixed in 10% buffered formalin. Standardized 5‐mm coronal slices were taken. The hippocampal CA1 sector and the cortex were chosen as regions of interest and were paraffin‐embedded. 14 , 15 , 16 Five‐micrometer‐thick sections were then obtained and stained with hematoxylin‐eosin. Three fields (at both ends and in the middle of CA1) at ×100 magnification (field of view diameter of 2 mm) were evaluated. In the cortex, 3 randomly selected fields at ×100 magnification (field of view diameter of 2 mm) were evaluated. The proportion of neuronal loss and degeneration/necrosis (shrunken neurons with deeply acidophilic cytoplasm and pyknotic nucleus) was quantified as absent (0), rare (1‐slight: <10% of pyramidal cells affected), few (2‐moderate: 10%–50% of pyramidal cells affected), and numerous (3‐severe: >50% of pyramidal cells affected). Serial sections were stained with Fluoro‐Jade (Sigma‐Aldrich) and examined with a fluorescence microscope to confirm the presence of neuronal degeneration/necrosis and to distinguish it from artifactual changes and dark neurons. 18 Immunohistochemistry with antibody against microglia‐specific ionized calcium‐binding adaptor molecule 1 (Iba1) was used to detect reactive microglia activation. The evaluation was performed in the pyramidal CA1 layer of the hippocampus. Three fields, corresponding to an area of 0.3 mm2 each, were chosen among the most positively stained (hot spots) and photographed. Digital microphotographs of Iba1‐immunostained sections were submitted to a semiautomated image analysis (ImageJ analysis program http://rsb.info.nih.gov/ij/). The area of reactive microglia was expressed as a percentage of Iba1‐positive stain. 14 , 15 , 16 An experienced board‐certified veterinary pathologist (E.S.) blinded to treatment made these assessments.
For transmission electron microscopy, hippocampus samples were reduced and fixed with 4% paraformaldehyde and 2% glutaraldehyde in phosphate buffer 0.12 mol/L pH 7.4 overnight at 4°C, followed by incubation at room temperature for 2 hours in 2% osmium tetroxide. After dehydration in a graded series of ethanol preparations, tissue samples were cleared in propylene oxide, embedded in epoxy medium (Epoxy Embedding Medium kit, Sigma‐Aldrich), and polymerized at 60°C for 72 hours. Ultra‐thin sections (60‐nm thick) of areas of interest were obtained with a Leica EM UC6 ultramicrotome (Leica Microsystems), counterstained with uranyl acetate and lead citrate and examined with an Energy Filter Transmission Electron Microscope (LIBRA 120, Carl Zeiss AG) equipped with a YAG scintillator slow scan charge‐coupled device camera. 15
Myocardial infarct was assessed by triphenyltetrazolium chloride staining. The left ventricle was sliced into 5‐mm‐thick transverse sections, which were incubated (20 minutes) in a solution of triphenyltetrazolium chloride and then transferred to 10% neutral buffered formalin overnight before image analysis. Infarct size was reported as the percentage of triphenyltetrazolium chloride–negative area relative to the LV area. 14 , 15 , 16
Statistical Analysis
A 1‐sample Kolmogorov–Smirnov Z test was used to confirm normal distribution of the data. For comparison of time‐based variables, 2‐way ANOVA with Tukey multiple comparisons test was used. Time‐based variables not normally distributed (neurological alertness score, neurological deficit score, Kyn metabolites, and hs‐cTnT) were transformed into the natural logarithm in order to satisfy the assumptions required by the models, then analyzed with 2‐way ANOVA with Tukey multiple comparisons test. For comparisons of variables with only a time point, 1‐way ANOVA with Tukey multiple comparison test was used for parametric variables, and the Kruskal‐Wallis test with Dunn multiple comparison was used for non‐normally distributed variables. When the dependent variable was categorical, chi‐square test for trend was used. Parametric variables are expressed as mean±SEM, and nonparametric variables are presented as median [quartile 1–quartile 3]. The sample size was estimated on the survival with good neurological recovery. Using the incidence of overall performance category 1 to 2, from a previous similar study (25% after 12 minutes of untreated CA), 16 and assuming an increase to 80% in the Ar 70% group compared with the control group, 12 animals per group would be needed to have a power=0.8 (α=0.05, 2‐sided). Statistical analyses were performed using GraphPad Prism 7.02 (GraphPad Software Inc). P≤0.05 was regarded as statistically significant.
Results
No differences were observed in body weight, heart rate, hemodynamics, EtCO2, myocardial function, arterial blood gases, and circulating biomarkers in the 3 groups at baseline (Tables 1 and 2, Figure 2).
Table 1.
Resuscitation Outcome and Hemodynamic Variables
| Control (n=12) | Ar 50% (n=12) | Ar 70% (n=12) | |
|---|---|---|---|
| Body weight, kg | 38±1 | 40±1 | 39±1 |
| Coronary perfusion pressure, mm Hg | |||
| CPR 1 min | 19±4 | 25±6 | 22±5 |
| CPR 3 min | 35±3 | 39±6 | 38±5 |
| CPR 5 min | 22±4 | 26±4 | 27±3 |
| Duration of CPR, s | 341 (301 to 366) | 372 (325 to 430) | 302 (301 to 359) |
| Total defibrillations delivered, n | 5 (2 to 12) | 18 (7 to 25) | 12 (7 to 15) |
| Successful resuscitation, n/total | 10/12 | 10/12 | 10/12 |
| 96‐h Survival, n/resuscitated | 6/10 | 7/10 | 9/10 |
| 96‐h Neuron‐specific enolase, ng/mL | 21 (16 to 61) | 8 (1 to 18) | 9 (3 to 22) |
| 96‐h Glial fibrillary acidic protein, pg/mL | 15 (7 to −21) | 8 (6 to 8) | 11 (6 to 13) |
| 96‐h Ubiquitin c‐terminal hydrolase L1, pg/mL | 64 (26 to 68) | 20 (18 to 43) | 24 (20 to 57) |
| Heart rate, beats per min | |||
| Baseline | 102±9 | 95±4 | 82±7 |
| Post‐resuscitation 2 h | 173±7 | 154±12 | 131±5* |
| Post‐resuscitation 4 h | 165±14 | 135±9 | 120±9* |
| Right atrial pressure, mm Hg | |||
| Baseline | 5±1 | 8±1 | 5±1 † |
| Post‐resuscitation 2 h | 7±1 | 8±1 | 6±1 |
| Post‐resuscitation 4 h | 7±1 | 9±1 | 7±1 |
| Oxygen, mm Hg | |||
| Baseline | 36±0 | 36±1 | 36±1 |
| Post‐resuscitation 2 h | 36±0 | 35±0 | 36±0 |
| Post‐resuscitation 4 h | 36±0 | 36±0 | 37±0 |
| Pulmonary arterial pressure, mm Hg | |||
| Baseline | 19±2 | 22±1 | 18±1 |
| Post‐resuscitation 2 h | 20±1 | 19±2 | 19±2 |
| Post‐resuscitation 4 h | 21±1 | 21±1 | 21±2 |
| Pulmonary capillary wedge pressure, mm Hg | |||
| Baseline | 9±1 | 11±1 | 9±0 |
| Post‐resuscitation 2 h | 11±1 | 12±1 | 9±1 |
| Post‐resuscitation 4 h | 12±1 | 12±1 | 10±1 |
| LV cardiac output, L/min | |||
| Baseline | 4.4±0.2 | 3.9±0.2 | 4.0±0.2 |
| Post‐resuscitation 2 h | 3.2±0.3 | 2.9±0.3 | 3.0±0.3 |
| Post‐resuscitation 4 h | 3.0±0.2 | 2.5±0.1 | 2.6±0.2 |
| LV ejection fraction, % | |||
| Baseline | 76.6±2.3 | 69.5±3.9 | 72.7±3.1 |
| Post‐resuscitation 2 h | 35.7±3.1 | 46.8±8.2 | 34.6±3.3 |
| Post‐resuscitation 4 h | 36.6±3.5 | 45.6±7.1 | 39.0±4.5 |
| Post‐resuscitation 96 h | 55.1±7.4 | 50.9±5.9 | 67.5±2.6 † |
| LV end‐diastolic volume, mL | |||
| Baseline | 33.7±3.0 | 31.5±1.8 | 39.5±3.5 |
| Post‐resuscitation 2 h | 35.4±3.3 | 27.3±2.3 | 31.5±2.9 |
| Post‐resuscitation 4 h | 42.5±5.7 | 36.6±6.1 | 39.4±2.6 |
| Post‐resuscitation 96 h | 41.3±4.3 | 36.2±2.4 | 37.1±4.8 |
| LV end‐systolic volume, mL | |||
| Baseline | 7.8±0.9 | 9.8±1.4 | 13.3±1.8 |
| Post‐resuscitation 2 h | 22.8±2.6 | 14.3±2.8 | 20.4±2.4 |
| Post‐resuscitation 4 h | 26.4±4.2 | 21.3±5.6 | 23.0±2.3 |
| Post‐resuscitation 96 h | 18.2±3.5 | 17.8±2.3 | 12.4±2.1 |
Data are reported as mean±SEM or median (interquartile range). Two‐way ANOVA with Tukey multiple comparison test was used for coronary perfusion pressure, heart rate, right atrial pressure, end‐tidal partial pressure of carbon dioxide, pulmonary arterial pressure, pulmonary capillary wedge pressure, left ventricular (LV) cardiac output and LV ejection fraction, end‐diastolic and systolic volumes (these last 3 parameters of myocardial function were echocardiographically assessed in 8, 9, and 9 animals in the control, argon 50% [Ar 50%], and argon 70% [Ar 70%] groups, respectively). Kruskal‐Wallis with Dunn multiple comparison test was used for the duration of cardiopulmonary resuscitation (CPR), total defibrillations delivered, and circulating biomarkers. One‐way ANOVA with Tukey multiple comparison was used for body weight, and chi‐square test for trend was used for successful resuscitation and 96‐hour survival.
P<0.01 vs control.
P<0.05 vs Ar 50%.
Table 2.
Arterial Blood Gases
| Control (n=12) | Ar 50% (n=12) | Ar 70% (n=12) | |
|---|---|---|---|
| pH | |||
| Baseline | 7.487±0.02 | 7.459±0.01 | 7.500±0.03 |
| Post‐resuscitation 2 h | 7.373±0.02 | 7.325±0.03 | 7.398±0.02 |
| Post‐resuscitation 4 h | 7.424±0.03 | 7.408±0.02 | 7.439±0.02 |
| Carbon dioxide partial pressure | |||
| Baseline | 38±1 | 37±1 | 36±1 |
| Post‐resuscitation 2 h | 40±1 | 38±1 | 40±1 |
| Post‐resuscitation 4 h | 40±1 | 39±1 | 40±1 |
| Oxygen partial pressure | |||
| Baseline | 80±4 | 83±3 | 88±4 |
| Post‐resuscitation 2 h | 110±6 | 113±7 | 112±8 |
| Post‐resuscitation 4 h | 103±8 | 111±8 | 114±9 |
| Base excess | |||
| Baseline | 5.4±1.4 | 2.3±0.7 | 4.8±1.3 |
| Post‐resuscitation 2 h | −1.8±1.6 | −6.1±1.3 | −0.1±1.4* |
| Post‐resuscitation 4 h | 2.3±1.5 | 0±1.2 | 3.0±1.1 |
| Bicarbonate | |||
| Baseline | 29±1 | 27±0.6 | 28±1 |
| Post‐resuscitation 2 h | 23±1 | 20±1.2 | 25±1* |
| Post‐resuscitation 4 h | 26±1 | 25±0.9 | 27±1 |
Data are mean±SEM. Two‐way ANOVA with Tukey multiple comparison test. Ar 70% indicates argon 70%.
P<0.01 vs argon 50% (Ar 50%).
Figure 2. Systolic blood pressure (SAP), mean arterial pressure (MAP), and diastolic arterial pressure (DAP) in argon (Ar)‐treated animals and controls at baseline and during the 4‐hour post‐resuscitation period.
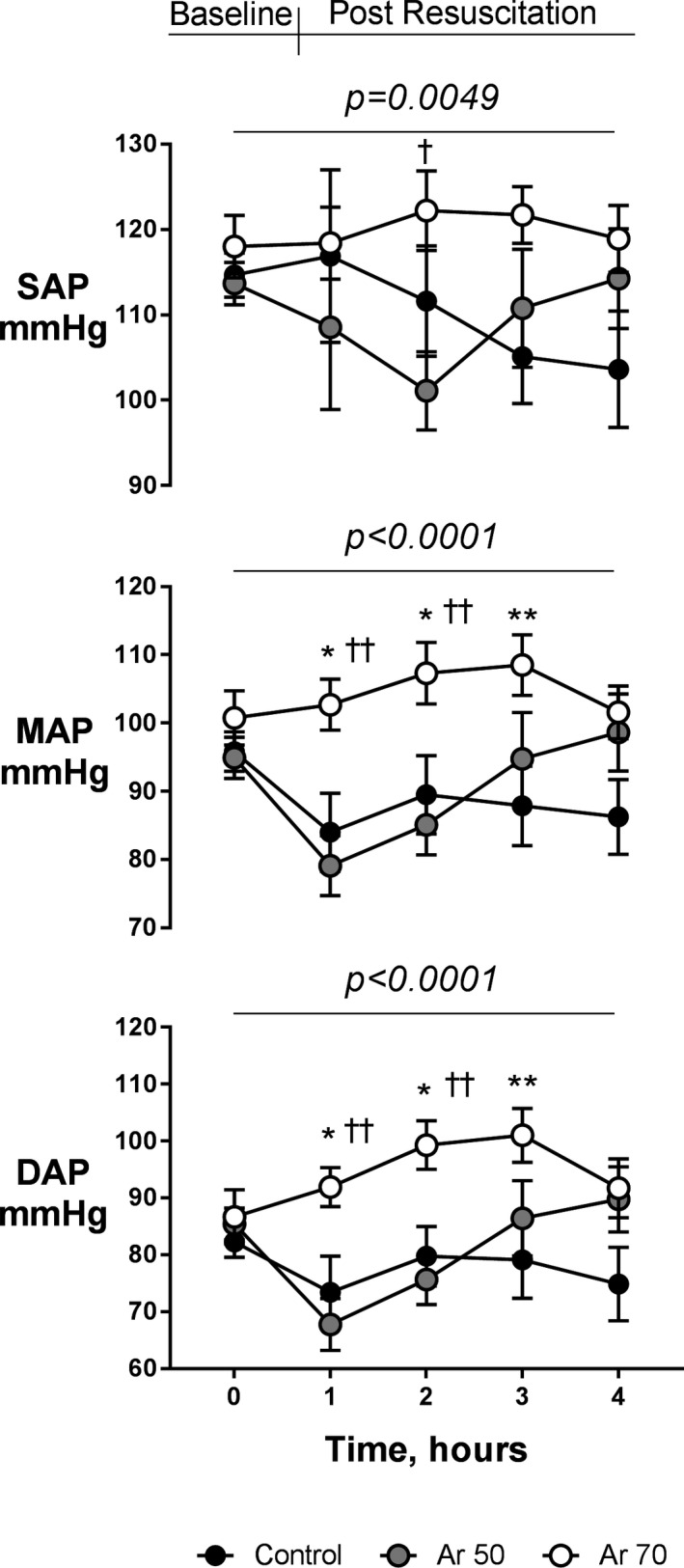
Mean±SEM. Two‐way ANOVA (P for treatment at the top of each panel) with Tukey multiple comparison test. *P<0.05 and **P<0.01 vs control; † P<0.05 and †† P<0.01 vs Ar 50%.
Ten animals in each group were successfully resuscitated (Table 1). Coronary perfusion pressure was similar in the 3 groups during the 5 minutes of CPR. No significant differences were observed among groups in the duration of CPR or in the total number of defibrillations delivered before final resuscitation, although there were more in Ar‐treated animals than controls (Table 1). Specifically, the Ar 50% animals were subjected to the largest number of defibrillations and the longest CPR before resuscitation and the start of the randomized experimental treatment.
Ar had a beneficial effect on heart rate and arterial pressure (P<0.0001 versus control). Animals in the Ar 70% group had a significantly lower heart rate 2 and 4 hours after resuscitation than those in the control group (P<0.01, Table 1). This was associated with significantly higher systolic, mean, and diastolic arterial pressures in animals treated with Ar 70% compared with those receiving Ar 50% and controls (P<0.01, Figure 2). No differences in cardiac output and other hemodynamic variables were seen among groups (Table 1).
Post‐resuscitation myocardial function was significantly impaired in all animals during the 4 hours of observation, with increased LV volumes and reduced LV ejection fraction (LVEF) (Table 1). However, animals treated with Ar 70% had a complete myocardial function recovery 96 hours after CA (Table 1). As shown in Data S1, from 4 to 96 hours after resuscitation, LVEF improved more in the Ar 70% group than in the Ar 50% and control groups, ie, 29%±6% versus 5%±7% and 19%±7%, respectively (Table S1). Moreover, significantly higher LVEF and completely restored LV systolic volume at 96 hours were observed in animals treated with Ar 70% (P<0.05 versus Ar 50%, Table 1).
Compared with controls, ventilation with Ar did not impair respiratory gas exchanges or acid‐base homeostasis (Table 2). At 2 hours post‐resuscitation, base excess and bicarbonate were lower in animals treated with Ar 50% than in those receiving Ar 70% (P<0.01, Table 2).
No significant difference among groups was observed in the numbers of animals that survived up to 96 hours, although there was a tendency toward better survival in the Ar group (survival of 60%, 70%, and 90% in the control, Ar 50%, and Ar 70% groups, respectively; Table 1).
Animals treated with Ar had significantly better neurological recovery than controls, again with a tendency in favor of higher Ar concentrations (Figures 3 and 4). Respectively, 60% and 80% of resuscitated animals in the Ar 50% and Ar 70% groups achieved complete neurological recovery 96 hours after resuscitation in contrast to only 30% in the control group (P<0.0001, Figure 3). Neurological alertness score and neurological deficit score were also significantly better in Ar‐treated animals than controls at 24, 48, and 72 hours after resuscitation (P<0.05, Figure 4). This was accompanied by significantly less kynurenine pathway activation, indicated by a lower kynurenine/tryptophan ratio, after Ar (P=0.026, Figure 5). Ar also reduced the release of neurotoxic 3‐hydroxyanthranilic acid (P=0.011), with circulating levels significantly lower in the Ar 70% group (P<0.05 versus control, Figure 5). A tendency toward lower circulating levels of neuron‐specific enolase, glial fibrillary acidic protein, and ubiquitin C‐terminal hydrolase L1 in the Ar groups compared with the control group was also observed (Table 1).
Figure 3. Ninety‐six‐hour survival with overall performance category (OPC) scores.
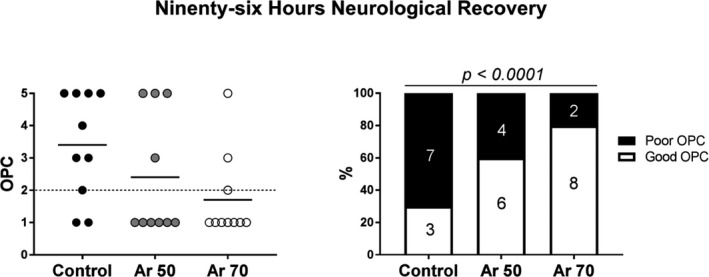
On the left are individual scores and means, and on the right, cumulative outcomes dichotomized in good (OPC ≤2) and poor (OPC ≥3). Chi‐square test for trend. Ar indicates argon.
Figure 4. Neurologic alertness scores and neurologic deficit scores in argon (Ar)‐treated animals and controls 24, 48, and 72 hours post‐resuscitation.
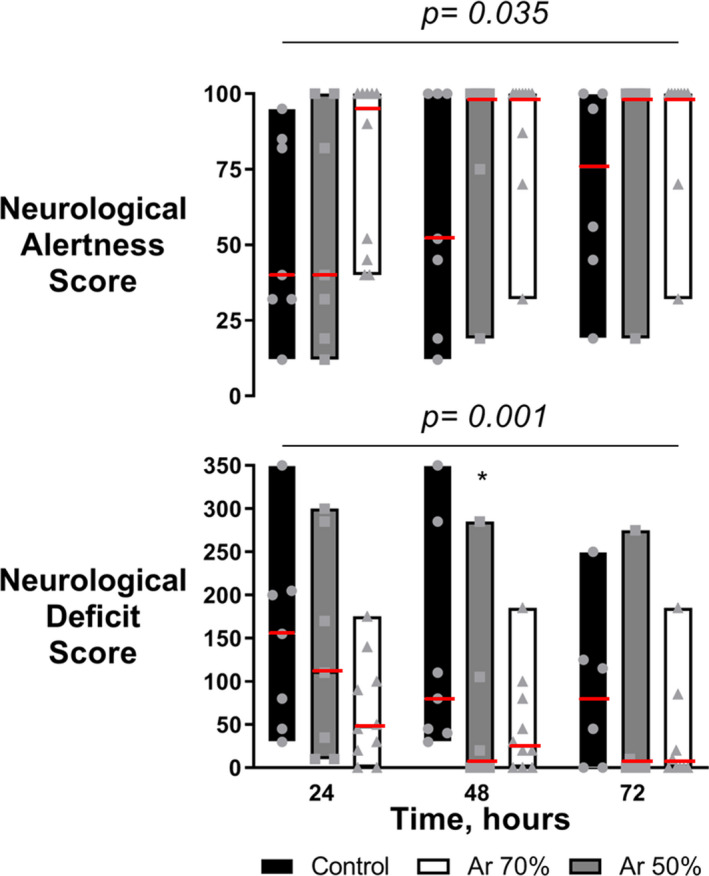
Median (red line) with interquartile range plus jittered dots for single animals. Two‐way ANOVA (P for treatment at the top of each panel) with Tukey multiple comparisons test on log‐transformed values. *P<0.05 vs control.
Figure 5. Plasma kynurenine/tryptophan ratio, kynurenic acid, and 3‐hydroxyanthranilic acid, at baseline (BL) and 4 hour post‐resuscitation.
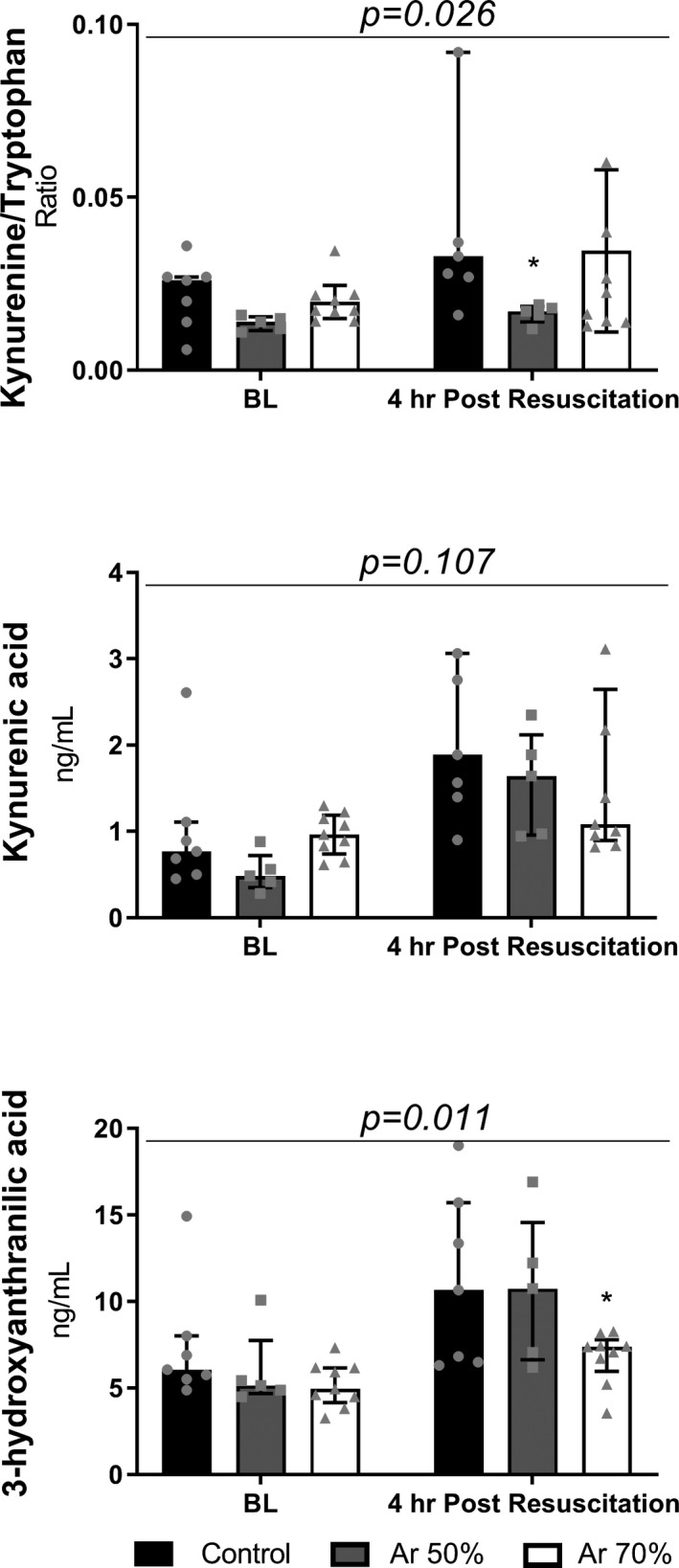
Median and interquartile range plus jittered dots for single animals. Two‐way ANOVA (P for treatment at the top of each panel) with Tukey multiple comparisons test on log‐transformed concentrations. *P<0.05 vs Control.
Brain histology showed a significant reduction of neuronal degeneration in the cortex (P<0.05), but not in the hippocampus, and less reactive microglial activation in the hippocampus (P=0.007) after treatment with Ar compared with control (Figure 6). Serial hippocampal sections stained with Fluoro‐Jade confirmed the presence of neuronal degeneration/necrosis observed with hematoxylin‐eosin staining (Figure 7). Ventilation with Ar 70% also preserved the mitochondrial ultrastructure in the hippocampus by reducing ischemia‐induced damage of cristae and reducing the matrix density compared with control ventilation (Figure 8).
Figure 6. Representative images of neuronal degeneration in brain hippocampal CA1 sector and cortex.
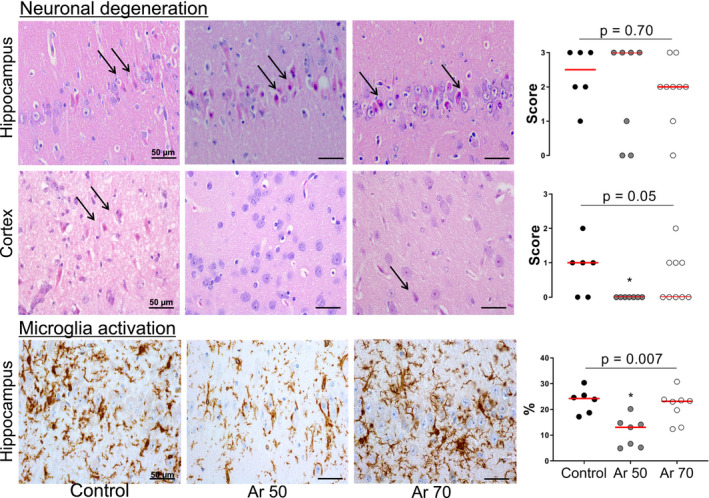
Hematoxylin‐eosin staining (original magnification ×400) showing intact neurons in argon (Ar)‐treated animals in contrast to ischemically damaged ones in controls (arrows). Microglial activation in brain hippocampal CA1 sector. Immunohistochemistry for microglia‐specific ionized calcium‐binding adaptor molecule 1 (original magnification ×400) showing typical morphology for normal resting microglia in Ar‐treated animals in contrast to activated microglia in a tract of pyramidal cells in controls. Data are shown as dot plots with median. Kruskal‐Wallis test with Dunn multiple comparisons test. *P<0.05 vs control.
Figure 7. Representative serial sections of brain hippocampal CA1 sector, stained with hematoxylin‐eosin (H & E, on the top) and Fluoro‐Jade B (on the bottom), in control and argon (Ar)‐treated animals.
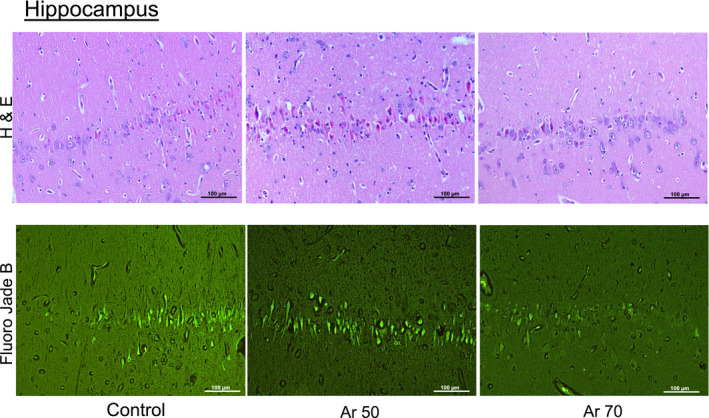
Original magnification ×200.
Figure 8. Ultrastructural analysis of hippocampus.
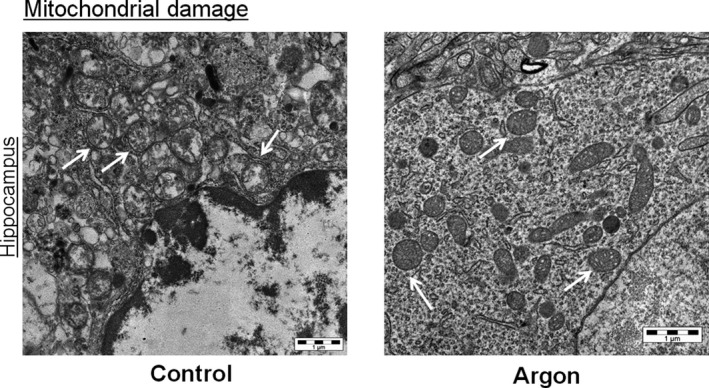
Representative transmission electron microscopy images of neurons (mitochondria indicated by white arrows). In the control animal, mitochondria show disruption of cristae and marked reduction of matrix density; in the animal treated with argon (Ar) 70%, mitochondria show only irregular shapes and moderate damage of inner membranes.
Gross heart anatomy showed a significant reduction in LV infarct size after Ar treatment compared with control (P=0.006, Figure 9), with a greater reduction in animals given Ar 70%. In all, plasma levels of hs‐cTnT were lower in animals treated with Ar compared with those in the control group (P=0.001), again with significantly lower levels in the Ar 70% group than in the Ar 50% and control groups (P<0.01 and P<0.05, respectively; Figure 9).
Figure 9. Top, Left ventricular infarct size, as the percentage of triphenyltetrazolium chloride–negative area relative to the left ventricular area in animals treated with argon (Ar) in comparison to controls (Kruskal‐Wallis test). Bottom, Plasma high‐sensitivity cardiac troponin T in Ar‐treated animals and controls, at baseline, during the 4‐hour post‐resuscitation period and 96 hours later.
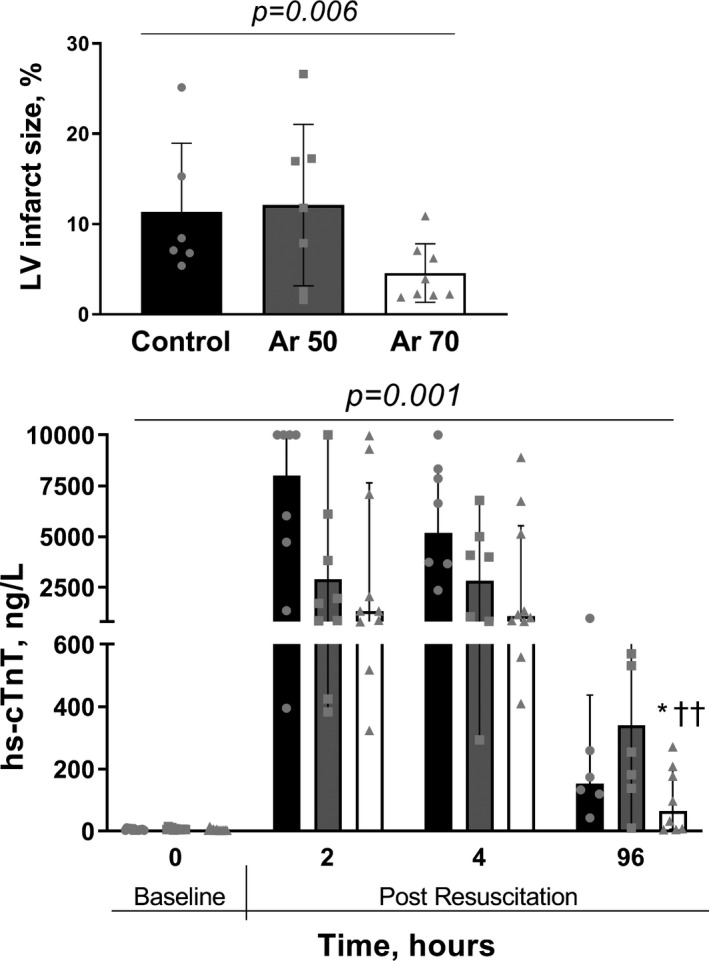
Two‐way ANOVA (P for treatment at the top) with Tukey multiple comparisons test on log‐transformed concentrations. Median and interquartile range plus jittered dots for single animals. *P<0.05 vs control; †† P<0.01 vs Ar 50%.
Discussion
This study indicates that 4 hours post‐resuscitation ventilation with Ar, initiated immediately after return of spontaneous circulation, significantly improved neurologic recovery after CA with a long no‐flow duration. More than twice of the animals treated with Ar survived 96 hours with good neurologic recovery, evaluated with different functional tests, in comparison to animals receiving control ventilation. Histopathology and circulating markers of brain injury supported the clinical benefits in Ar‐treated animals. In addition, a dose dependency of this Ar‐induced neuroprotection emerged from this study. Ar 70% also gave myocardial protection, represented by a smaller infarct and complete LVEF recovery at 96 hours after CA, coupled with better hemodynamics and less cardiac troponin release during the post‐resuscitation period. Finally, no detrimental effects were observed on respiratory function and gas exchange.
Several experimental studies have explored the neuroprotective properties of ventilation with Ar after CA. 11 , 12 , 13 , 14 , 19 Brücken and colleagues 12 first investigated Ar‐induced neuroprotection in a rat model of CA and CPR. After 7 minutes of no‐flow, the effect of ventilation with a mixture of Ar 70% in oxygen, starting 1 hour after resuscitation, led to better neurological recovery, evaluated daily during the week after CA, compared with the control ventilation. This was paralleled by significantly less damage in the neocortex and the hippocampal CA 3 and 4 regions. Additional studies from the same group showed that Ar‐induced neuroprotection persisted even when the onset of the treatment was delayed up to 3 hours after resuscitation. 13 Concurrently, our group has reported that 4‐hour post‐resuscitation treatment with Ar 70% in oxygen, initiated immediately after return of spontaneous circulation, gave faster and complete neurologic recovery, with no detrimental effects on hemodynamics and respiratory gas exchanges, in a porcine model of CA with intermediate no‐flow duration, ie, 8 minutes. 14 The efficacy of such a 4‐hour treatment with inhalatory Ar in preserving the brain from ischemic injury has now been proven in this more severe, clinically relevant porcine model of CA.
Clinical studies have reported an average emergency medical service arrival time to the CA scene of 6 to 8 minutes; however, considering the time taken for emergency call, ambulance dispatch, arrival to the patient, and onset of CPR, it is reasonable to assume that the no‐flow may be longer. Thus, untreated CA in experimental models lasting more than 8 minutes adequately reflects the clinical environment. 20 , 21 In our model, data on survival resembled those reported clinically, only when the duration of no‐flow exceeded 12 minutes, meaning the results can easily be transferred to humans. 15 , 16
The dose dependency of Ar effects is controversial. An earlier study in rats showed a significantly better neurological outcome and histopathological brain injury after ventilation with Ar at either 40% or 70% after resuscitation, in contrast to ventilation without Ar. However, the neuroprotective effects were significantly more pronounced when the inhalatory mixture contained 70% Ar. 22 In contrast, a tendency toward less neuronal damage in animals subjected to 24 hours of ventilation with Ar 50% after resuscitation compared with controls was reported in another model of CA in rodents. 19 The present study, in a preclinically relevant model, indicates that Ar exerts a greater beneficial effect at higher concentrations. Treatment with Ar 50% provided better neurological recovery together with less histopathological brain injury than the control treatment. However, Ar 70% achieved further improvement in survival with complete neurological recovery, confirming that the higher Ar concentration should be preferred to preserve the brain better after CA. Circulating levels of the neurotoxic marker 3‐hydroxyanthranilic acid, a downstream metabolite of the kynurenine pathway, associated with neurological damage and poor outcome after CA, 17 , 23 support the greater neuroprotective effect of Ar 70% compared with Ar 50%. However, Ar at concentrations <70% need to be investigated further, since this might be considered useful in patients requiring fraction of inspired oxygen >30% to achieve and/or maintain adequate arterial oxygen saturation. We are aware that the improvements in neuronal degeneration after Ar treatment were not evident in the hippocampus, and this is in contrast with the concurrent significant reduction in microglia activation observed in the same area. One reason might be the relatively small sample of brains harvested and analyzed, attributable to many resuscitated animals (40%) in the control group dying in the first days after resuscitation. These early deaths might have excluded more injured animals with probably greater brain damage. However, we cannot exclude that the selection of the hippocampal CA1 sector for histopathology was not the most appropriate. Similar models of CA in the pig, in fact, caused histological detectable brain damage that was more evident in other areas, ie, putamen and caudate nucleus, whereas they were to a lesser extent in the CA1. 24 , 25 The 4‐day interval between CA and histopathological assessment was also probably too short, since the maximal injury in this specific brain area has been reported to occur around the seventh day post‐resuscitation, because of delayed neuronal cell death. 26 Finally, in addition to brain injury, microglia is also known to be activated and modulated in response to other extracellular stimuli, ie, proinflammatory triggers 27 and kynurenine metabolites. 28 Nevertheless, it is noteworthy that functional outcome revealed significant differences among groups, with a clear improvement in overall neurologic performance in Ar‐treated animals.
Our previous study in the 8‐minute CA model in the pig indicated that ventilation with Ar 70% was associated with a tendency toward a smaller LV infarct and less hs‐cTnT release compared with control ventilation. 14 Cardioprotection by Ar has also been described in other models of myocardial ischemia/reperfusion in rodents, which provided evidence of a reduced infarct size and improved LV systolic function, evaluated by magnetic resonance. 29 , 30 The present study, beside neuroprotection, also confirmed the Ar‐mediated cardioprotective effects. Ventilation with Ar 70% yielded significantly better post‐resuscitation myocardial function, with significantly greater arterial pressure, smaller infarct, and less troponin release, together with evidence of a complete recovery of LVEF, compared with ventilation with Ar 50%. Nevertheless, it cannot be excluded that the longer duration of CPR and the larger number of defibrillations delivered in the Ar 50% group compared with the Ar 70% group might have accounted for the worse post‐resuscitation myocardial function and less beneficial effect on final outcome compared with ventilation with a higher Ar concentration.
Although noble gases are traditionally believed to be inert, several potential mechanisms of action involved in the Ar protection have been reported. Ar appeared to possess oxygen‑like properties, which could explain its neuroprotective action, partially restoring mitochondrial respiratory enzyme activity and reducing N‑methyl‑D‑aspartic acid–induced neuronal death. 11 , 31 Ar has antiapoptotic effects on the molecular pathways involved in cell survival: it increases extracellular signal‑regulated kinase (ERK) 1/2 phosphorylation; blocks the apoptosis cascade 32 ; upregulates the expression of the antiapoptotic protein B‑cell lymphoma‑2 33 ; activates the toll‑like receptor 2 and 4, reducing caspase‑3 activity 34 ; and mediates the intracellular signaling involved in the production of proinflammatory cytokines, growth factors, and cell survival. 35 In addition, at reperfusion, Ar has been reported to prevent the reperfusion‐induced mitochondrial permeability transition pore (MPTP) opening, thus preventing mitochondrial calcium‑phosphate overload, dissipation of mitochondrial membrane potential, and subsequent cell death. 29 , 30 Phosphatidylinositol‑3‑kinase and ERK 1/2 inhibit MPTP opening by their actions on several downstream signaling molecules that modulate the transition state of the pore either directly (via endothelial nitric oxide synthase, p53, glycogen synthase kinase) or indirectly by affecting the proapoptotic and antiapoptotic B‑cell lymphoma protein. Thus, by acting on the same pathways, directly or indirectly, through reperfusion injury salvage kinase, Ar prevents the MPTP opening. 11 , 29 , 30 Since, MPTP is a nonselective channel of the inner mitochondrial membrane that opens at reperfusion after prolonged ischemia, interventions protecting from MPTP opening after CA, such as Ar inhalation, ideally should be initiated as soon as possible after return of spontaneous circulation, as proposed in this investigation. Indeed, this study confirmed the Ar‐induced mitochondrial ultrastructure protection in the hippocampus, with less ischemia‐induced damage of cristae and matrix density compared with control ventilation. Finally, other studies have also suggested that Ar, by triggering γ‐aminobutyric acid neurotransmission, might lead to a decrease in N‐methyl‐D‐aspartate receptor stimulation, involved in postischemic excitotoxicity. 36
Importantly, this study confirmed earlier evidence on the safety of ventilation with Ar at a high concentration, even after resuscitation from a long CA. No detrimental effects have been reported on hemodynamics, respiratory function, and gas exchange. In addition, earlier data from our laboratory confirmed that prolonged ventilation with a mixture of Ar 70%–oxygen 30% in healthy pigs was safe. 37 In that investigation, no adverse events occurred during or after Ar ventilation. Hemodynamics, blood gas variables, hemoglobin, hematocrit, serum electrolytes, hs‐cTnT, neuron‐specific enolase, transaminases, and creatinine all remained stable in the physiological range. Finally, because Ar is denser than air, it could be argued that ventilation with this gas could increase respiratory resistance 38 ; however, none of the preclinical studies have reported this, even after prolonged exposure.
From all of the evidence previously reported in different models of CA and confirmed in this preclinically relevant porcine model of severe CA, the neuroprotective properties of ventilation with Ar should now be examined in the clinical scenario. Indeed, this study adds new insights to optimize the clinical use of this innovative treatment. The results support a dose‐dependent neuroprotective effect of early Ar administration, in the absence of any toxic or adverse reaction after a 4‐hour inhalation. Moreover, the successful and safe use of a clinical ventilator modified to deliver different Ar concentrations was reported. Thus, 4 hours of ventilation with high Ar concentration, ie, 70% in oxygen or 50%, if patients require higher fraction of inspired oxygen, initiated early after return of spontaneous circulation, ie, at hospital admission or even earlier in the ambulance, might be the base to design a first phase 2 clinical trial.
Other noble gases, especially xenon, have been investigated for neuroprotection after CA and have been already translated clinically. 10 , 11 Thus, in a recent randomized phase 2 clinical trial, 24 hours of xenon ventilation combined with TTM reduced white matter damage as measured by fractional anisotropy of diffusion tensor magnetic resonance imaging in comatose patients post‐CA. 10 A secondary analysis of the same trial also showed a less severe myocardial injury as demonstrated by the significantly lower levels of troponin‐T in patients given xenon plus TTM in comparison to TTM alone. 39 Compared with xenon, Ar has many advantages; at normobaric pressure, Ar has no hemodynamic properties and no hypnotic and anesthetic effects. Thus, it can be administered through an open‐circuit system, ie, a bag‐valve or a ventilator modified to be compatible with the gas mixture Ar–oxygen, allowing for potential early application during the initial post‐resuscitation phase or even in the prehospital setting. Finally, since Ar is ubiquitous in the atmosphere and available by extraction from liquefied air, the cost should be relatively low and sustainable, around 9 Euro cents per liter, ≈100 times lower than xenon. 10 , 14 Ar may thus appear an attractive clinical option for post‐CA syndrome.
We recognize the limitations in the interpretation of our findings. First, this was a purely descriptive study. Second, the effects of Ar were not compared with those of other noble gases. However, earlier investigations have reported similar neuroprotective effects of Ar and the widely studied xenon. 40 Third, the combination of Ar with target temperature management was not assessed. Nevertheless, earlier reports on combinations of Ar with target temperature management proved there were in fact no additional benefits from the combined treatment. 41 Finally, Ar pharmacokinetics was not investigated; however, pharmacokinetic models for humans, pigs, mice, and rats have already been reported. 42
Conclusions
The above limitations notwithstanding, in this severe model of CA and CPR, post‐resuscitation ventilation with Ar improved survival with good neurological recovery compared with control ventilation, with no detrimental effects on hemodynamics and respiratory gas exchanges. Neuroprotection was more pronounced after treatment with Ar 70% than Ar 50%. Finally, Ar 70% also showed cardioprotective effects after CA. Post‐resuscitation neuroprotective ventilation with a mixture of Ar 70%–oxygen 30% has been extensively investigated in animal models, confirming its safety and efficacy, and is now ready to be clinically translated.
Sources of Funding
The study was supported by a competitive grant to Ristagno, awarded from the Ministry of Health, Ricerca Finalizzata, Bando Giovani Ricercatori, grant agreement no. GR‐2011‐02348099. Fumagalli’s PhD scholarship was supported in part by the “Associazione Amici del Mario Negri.” Fondazione Sestini and SIAD, Bergamo, Italy, provided extra funding support.
Disclosures
None.
Supporting information
Data S1
Table S1
Acknowledgments
The authors thank Physio‐Control now part of Stryker, for the LUCAS 2 compressor; and Philips Medical Systems for the MRx defibrillator.
(J Am Heart Assoc. 2020;9:e016494. DOI: 10.1161/JAHA.120.016494.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016494
For Sources of Funding and Disclosures, see page 14.
References
- 1. Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, Regan S, Long J, Slowther A, Pocock H, et al. A randomized trial of epinephrine in out‐of‐hospital cardiac arrest. N Engl J Med. 2018;379:711–721. DOI: 10.1056/NEJMoa1806842. [DOI] [PubMed] [Google Scholar]
- 2. Okubo M, Schmicker RH, Wallace DJ, Idris AH, Nichol G, Austin MA, Grunau B, Wittwer LK, Richmond N, Morrison LJ, et al. Variation in survival after out‐of‐hospital cardiac arrest between emergency medical services agencies. JAMA Cardiol. 2018;3:989–999. DOI: 10.1001/jamacardio.2018.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gräsner JT, Lefering R, Koster RW, Masterson S, Böttiger BW, Herlitz J, Wnent J, Tjelmeland IB, Ortiz FR, Maurer H, et al. EuReCa ONE‐27 Nations, ONE Europe, ONE Registry: a prospective one month analysis of out‐of‐hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016;105:188–195. DOI: 10.1016/j.resuscitation.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 4. Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, Inter American Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. DOI: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 5. Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VRM, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post‐resuscitation care 2015: section 5 of the European Resuscitation Council guidelines for resuscitation 2015. Resuscitation. 2015;95:202–222. DOI: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 6. Cronberg T, Lilja G, Horn J, Kjaergaard J, Wise MP, Pellis T, Hovdenes J, Gasche Y, Åneman A, Stammet P, et al. Neurologic function and health‐related quality of life in patients following targeted temperature management at 33°C vs 36°C after out‐of‐hospital cardiac arrest: a randomized clinical trial. JAMA Neurol. 2015;72:634–641. DOI: 10.1001/jamaneurol.2015.0169. [DOI] [PubMed] [Google Scholar]
- 7. Lilja G, Nielsen N, Friberg H, Horn J, Kjaergaard J, Nilsson F, Pellis T, Wetterslev J, Wise MP, Bosch F, et al. Cognitive function in survivors of out‐of‐hospital cardiac arrest after target temperature management at 33°C versus 36°C. Circulation. 2015;131:1340–1349. DOI: 10.1161/CIRCULATIONAHA.114.014414. [DOI] [PubMed] [Google Scholar]
- 8. Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. DOI: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 9. Lascarrou J‐B, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, Coupez E, Dequin P‐F, Cariou A, Boulain T, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381:2327–2337. DOI: 10.1056/NEJMoa1906661. [DOI] [PubMed] [Google Scholar]
- 10. Laitio R, Hynninen M, Arola O, Virtanen S, Parkkola R, Saunavaara J, Roine RO, Grönlund J, Ylikoski E, Wennervirta J, et al. Effect of inhaled xenon on cerebral white matter damage in comatose survivors of out‐of‐hospital cardiac arrest: a randomized clinical trial. JAMA. 2016;315:1120–1128. DOI: 10.1001/jama.2016.1933. [DOI] [PubMed] [Google Scholar]
- 11. Nespoli F, Redaelli S, Ruggeri L, Fumagalli F, Olivari D, Ristagno G. A complete review of preclinical and clinical uses of the noble gas argon: evidence of safety and protection. Ann Card Anaesth. 2019;22:122–135. DOI: 10.4103/aca.ACA_111_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brücken A, Cizen A, Fera C, Meinhardt A, Weis J, Nolte K, Rossaint R, Pufe T, Marx G, Fries M. Argon reduces neurohistopathological damage and preserves functional recovery after cardiac arrest in rats. Br J Anaesth. 2013;110:i106–i112. DOI: 10.1093/bja/aes509. [DOI] [PubMed] [Google Scholar]
- 13. Brücken A, Kurnaz P, Bleilevens C, Derwall M, Weis J, Nolte K, Rossaint R, Fries M. Delayed argon administration provides robust protection against cardiac arrest‐induced neurological damage. Neurocrit Care. 2015;22:112–120. DOI: 10.1007/s12028-014-0029-1. [DOI] [PubMed] [Google Scholar]
- 14. Ristagno G, Fumagalli F, Russo I, Tantillo S, Zani DD, Locatelli V, De Maglie M, Novelli D, Staszewsky L, Vago T, et al. Postresuscitation treatment with argon improves early neurological recovery in a porcine model of cardiac arrest. Shock. 2014;41:72–78. DOI: 10.1097/SHK.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 15. Babini G, Grassi L, Russo I, Novelli D, Boccardo A, Luciani A, Fumagalli F, Staszewsky L, Fiordaliso F, De Maglie M, et al. Duration of untreated cardiac arrest and clinical relevance of animal experiments: the relationship between the "No‐Flow" duration and the severity of post‐cardiac arrest syndrome in a porcine model. Shock. 2018;49:205–212. DOI: 10.1097/SHK.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 16. Babini G, Ristagno G, Boccardo A, De Giorgio D, De Maglie M, Affatato R, Ceriani S, Zani D, Novelli D, Staszewsky L, et al. Effect of mild hypercapnia on outcome and histological injury in a porcine post cardiac arrest model. Resuscitation. 2019;135:110–117. DOI: 10.1016/j.resuscitation.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 17. Ristagno G, Latini R, Vaahersalo J, Masson S, Kurola J, Varpula T, Lucchetti J, Fracasso C, Guiso G, Montanelli A, et al. Early activation of the kynurenine pathway predicts early death and long‐term outcome in patients resuscitated from out‐of‐hospital cardiac arrest. J Am Heart Assoc. 2014;3:e001094. DOI: 10.1161/JAHA.114.001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wohlsein P, Deschl U, Baumgartner W. Nonlesions, unusual cell types, and postmortem artifacts in the central nervous system of domestic animals. Vet Pathol. 2013;50:122–143. DOI: 10.1177/0300985812450719. [DOI] [PubMed] [Google Scholar]
- 19. Zuercher P, Springe D, Grandgirard D, Leib SL, Grossholz M, Jakob S, Takala J, Haenggi M. A randomized trial of the effects of the noble gases helium and argon on neuroprotection in a rodent cardiac arrest model. BMC Neurol. 2016;16:43. DOI: 10.1186/s12883-016-0565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, Stiell I, Morrison LJ, Aufderheide TP, Cheskes S, et al. Trial of continuous or interrupted chest compressions during CPR. N Engl J Med. 2015;373:2203–2214. DOI: 10.1056/NEJMoa1509139. [DOI] [PubMed] [Google Scholar]
- 21. Hasselqvist‐Ax I, Riva G, Herlitz J, Rosenqvist M, Hollenberg J, Nordberg P, Ringh M, Jonsson M, Axelsson C, Lindqvist J, et al. Early cardiopulmonary resuscitation in out‐of‐hospital cardiac arrest. N Engl J Med. 2015;372:2307–2315. DOI: 10.1056/NEJMoa1405796. [DOI] [PubMed] [Google Scholar]
- 22. Brücken A, Kurnaz P, Bleilevens C, Derwall M, Weis J, Nolte K, Rossaint R, Fries M. Dose dependent neuroprotection of the noble gas argon after cardiac arrest in rats is not mediated by K(ATP)‐channel opening. Resuscitation. 2014;85:826–832. DOI: 10.1016/j.resuscitation.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 23. Ristagno G, Fries M, Brunelli L, Fumagalli F, Bagnati R, Russo I, Staszewsky L, Masson S, Li Volti G, Zappalà A, et al. Early kynurenine pathway activation following cardiac arrest in rats, pigs, and humans. Resuscitation. 2013;84:1604–1610. DOI: 10.1016/j.resuscitation.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 24. Fries M, Nolte KW, Coburn M, Rex S, Timper A, Kottmann K, Siepmann K, Häusler M, Weis J, Rossaint R, et al. Xenon reduces neurohistopathological damage and improves the early neurological deficit after cardiac arrest in pigs. Crit Care Med. 2008;36:2420–2426. DOI: 10.1097/CCM.0b013e3181802874. [DOI] [PubMed] [Google Scholar]
- 25. Fries M, Nolte K, Demir F, Kottmann K, Timper A, Coburn M, Weis J, Rossaint R. Neurocognitive performance after cardiopulmonary resuscitation in pigs. Crit Care Med. 2008;36:842–847. DOI: 10.1097/CCM.0B013E3181653041. [DOI] [PubMed] [Google Scholar]
- 26. Kudo Y, Ohtaki H, Dohi K, Yin LI, Nakamachi T, Endo S, Yofu S, Hiraizumi Y, Miyaoka H, Shioda S, et al. Neuronal damage in rat brain and spinal cord after cardiac arrest and massive hemorrhagic shock. Crit Care Med. 2006;34:2820–2826. DOI: 10.1097/01.CCM.0000242522.48734.64. [DOI] [PubMed] [Google Scholar]
- 27. Lull ME, Block ML. Microglia activation and chronic neurodegeneration. Neurotherapeutics. 2010;7:354–365.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garrison AM, Parrott JM, Tuñon A, Delgado J, Redus L, O'Connor JC. Kynurenine pathway metabolic balance influences microglia activity: targeting kynurenine monooxygenase to dampen neuroinflammation. Psychoneuroendocrinology. 2018;94:1–10. DOI: 10.1016/j.psyneuen.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lemoine S, Blanchart K, Souplis M, Lemaitre A, Legallois D, Coulbault L, Simard C, Allouche S, Abraini JH, Hanouz JL, et al. Argon exposure induces postconditioning in myocardial ischemia‐reperfusion. J Cardiovasc Pharmacol Ther. 2017;22:564–573. DOI: 10.1177/1074248417702891. [DOI] [PubMed] [Google Scholar]
- 30. Pagel PS, Krolikowski JG, Shim YH, Venkatapuram S, Kersten JR, Weihrauch D, Warltier DC, Pratt PF Jr. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth Analg. 2007;105:562–569. DOI: 10.1213/01.ane.0000278083.31991.36. [DOI] [PubMed] [Google Scholar]
- 31. David HN, Haelewyn B, Degoulet M, Colomb DG Jr, Risso JJ, Abraini JH. Ex vivo and in vivo neuroprotection induced by argon when given after an excitotoxic or ischemic insult. PLoS One. 2012;7:e30934. DOI: 10.1371/journal.pone.0030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ulbrich F, Kaufmann KB, Coburn M, Lagrèze WA, Roesslein M, Biermann J, Buerkle H, Loop T, Goebel U. Neuroprotective effects of argon are mediated via an ERK‐1/2 dependent regulation of heme‐oxygenase‐1 in retinal ganglion cells. J Neurochem. 2015;134:717–727. DOI: 10.1111/jnc.13115. [DOI] [PubMed] [Google Scholar]
- 33. Zhao H, Mitchell S, Koumpa S, Cui YT, Lian Q, Hagberg H, Johnson MR, Takata M, Ma D. Heme oxygenase‐1 mediates neuroprotection conferred by argon in combination with hypothermia in neonatal hypoxia‐ischemia brain injury. Anesthesiology. 2016;125:180–192. DOI: 10.1097/ALN.0000000000001128. [DOI] [PubMed] [Google Scholar]
- 34. Ulbrich F, Kaufmann K, Roesslein M, Wellner F, Auwärter V, Kempf J, Loop T, Buerkle H, Goebel U. Argon mediates anti‐apoptotic signaling and neuroprotection via inhibition of toll‐like receptor 2 and 4. PLoS One. 2015;10:e0143887. DOI: 10.1371/journal.pone.0143887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ulbrich F, Goebel U. The molecular pathway of argon‐mediated neuroprotection. Int J Mol Sci. 2016;17:E1816. DOI: 10.3390/ijms17111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abraini H, Kriem B, Balon N, Rostain JC, Risso JJ. gamma‐Aminobutyric acid neuropharmacological investigations on narcosis produced by nitrogen, argon, or nitrous oxide. Anesth Analg. 2003;96:746–749. DOI: 10.1213/01.ANE.0000050282.14291.38. [DOI] [PubMed] [Google Scholar]
- 37. Cucino A, Ruggeri L, Olivari D, De Giorgio D, Latini R, Ristagno G. Safety of ventilation with an argon and oxygen gas mixture. Br J Anaesth. 2019;122:e31–e32. DOI: 10.1016/j.bja.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 38. Arola O, Saraste A, Laitio R, Airaksinen J, Hynninen M, Bäcklund M, Ylikoski E, Wennervirta J, Pietilä M, Roine RO, et al. Inhaled xenon attenuates myocardial damage in comatose survivors of out‐of‐hospital cardiac arrest: the Xe‐Hypotheca trial. J Am Coll Cardiol. 2017;70:2652–2660. DOI: 10.1016/j.jacc.2017.09.1088. [DOI] [PubMed] [Google Scholar]
- 39. Behnke AR, Yorarbrough OD. Respiratory resistance, oil‐water solubility and mental effects of argon compared with helium and nitrogen. Am J Physiol. 1939;126:409–415. DOI: 10.1152/ajplegacy.1939.126.2.409. [DOI] [Google Scholar]
- 40. Jawad N, Rizvi M, Gu J, Adeyi O, Tao G, Maze M, Ma D. Neuroprotection (and lack of neuroprotection) afforded by a series of noble gases in an in vitro model of neuronal injury. Neurosci Lett. 2009;460:232–236. DOI: 10.1016/j.neulet.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 41. Brücken A, Bleilevens C, Föhr P, Nolte K, Rossaint R, Marx G, Fries M, Derwall M. Influence of argon on temperature modulation and neurological outcome in hypothermia treated rats following cardiac arrest. Resuscitation. 2017;117:32–39. DOI: 10.1016/j.resuscitation.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 42. Katz I, Murdock J, Palgen M, Pype J, Caillibotte G. Pharmacokinetic analysis of the chronic administration of the inert gases Xe and Ar using a physiological based model. Med Gas Res. 2015;5:8. DOI: 10.1186/s13618-015-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1


