Figure 1.
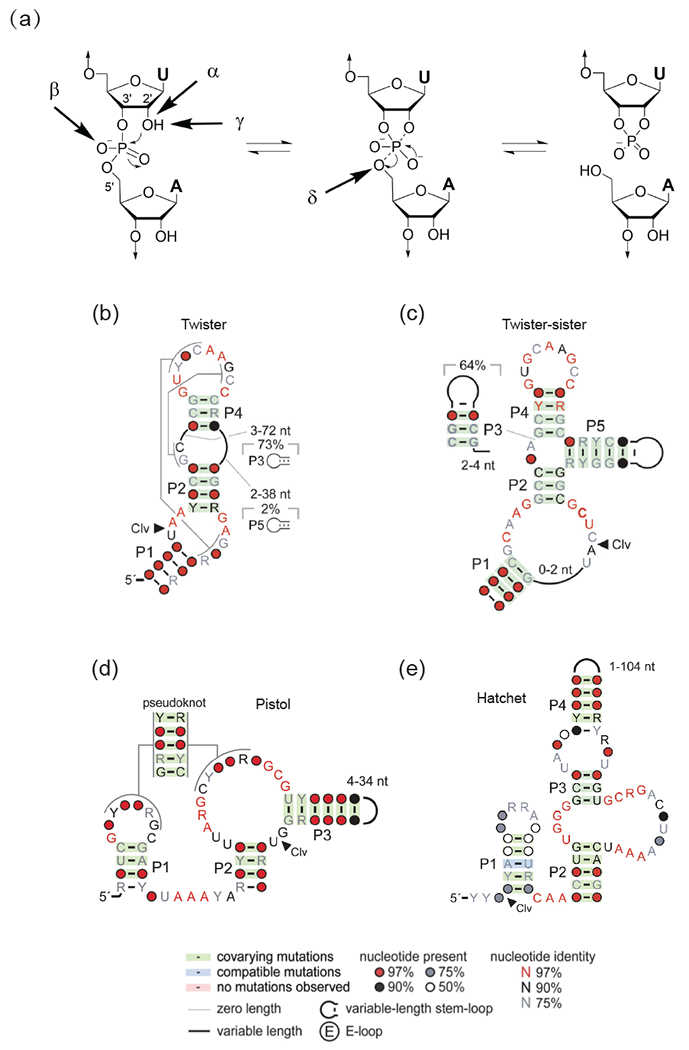
Catalytic strategies for cleavage of the phosphodiester backbone by self-cleaving ribozymes, as well as consensus sequences and secondary structures of newly-identified self-cleaving twister, twister-sister, pistol and hatchet ribozymes.
(a) Four factors that can contribute to catalysis during internal phosphodiester transfer reaction. α factor: in-line alignment of the 2’-O, phosphorus and O-5’ at the scissile phosphate; β factor: neutralization of the developing negative charge on the nonbridging phosphate oxygen; γ factor: general base-mediated deprotonation of the 2’-O; δ factor: general acid-based neutralization of the developing negative charge in the O-5’ position. (adopted from [19]). (b-e) Consensus sequence and secondary structures of newly-identified self-cleaving twister (panel b), twister-sister (panel c), pistol (panel d) and hatchet (panel e) ribozymes (adopted from [26•, 51]).
