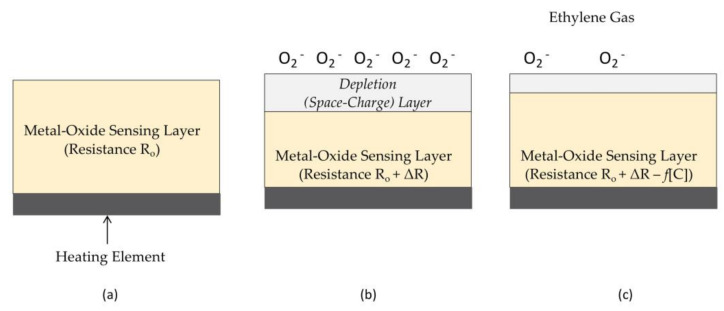
Figure 6.
Metal-Oxide Gas Sensors. (a) In the absence of oxygen and reducing gases, the metal oxide has a baseline resistance on the order of kOhms. (b) Ambient oxygen on the surface of the sensing layer extracts oxygen from the underlying metal oxide, increasing resistance and creating a depletion (insulating) layer on the surface of the sensor. (c) A reducing gas such as ethylene binds with the oxygen on the surface, resulting in a re-injection of electrons into the sensing layer and a decrease in resistance that is a function of the ambient ethylene concentration. The heating element keeps the metal oxide sensing layer at an elevated temperature that maximizes the sensitivity of the sensor to desired gases.