Abstract
Often blamed for bringing green aromas and astringency to wines, the use of stems is also empirically known to improve the aromatic complexity and freshness of some wines. Although applied in different wine-growing regions, stems use remains mainly experimental at a cellar level. Few studies have specifically focused on the compounds extracted from stems during fermentation and maceration and their potential impact on the must and wine matrices. We identified current knowledge on stem chemical composition and inventoried the compounds likely to be released during maceration to consider their theoretical impact. In addition, we investigated existing studies that examined the impact of either single stems or whole clusters on the wine quality. Many parameters influence stems’ effect on the wine, especially grape variety, stem state, how stems are incorporated, when they are added, and contact duration. Other rarely considered factors may also have an impact, including vintage and ripening conditions, which could affect the lignification of the stem.
Keywords: grape stems, whole clusters, wine, winemaking practice, phenolic compounds, antioxidant activity, maceration technique, polyphenolic compounds
1. Introduction
For white winemaking, stems are generally kept during pressing because they allow for better juice extraction yields. Given the short contact time, compounds are extracted from the stems in relatively low levels. In red winemaking, the maceration phase—where color is extracted from the grape skin and tannins from the grape seeds—occurs before pressing. Originally, stems were kept during this phase, but destemming practices appeared at the end of the 19th century, improving wine quality by reducing excessive astringency and negative strong green tastes from the stems [1]. Initially used in cellars with high production capacity, the first destemming machines quickly trivialized this practice; today, this technique is systematic for winegrowers in most wine-producing countries [2]. However, in some regions, using whole clusters of grapes is a matter of tradition. The stem is considered a natural additive that, if well mastered, brings complexity, freshness, and phenolic structure to the wine and facilitates chemical stability during aging [3] (e.g., the Pinot Noir in Burgundy, the Cabernet Franc in the Loire Valley, or the Gamay in the Beaujolais, Kakhethian wines from Georgia, etc.). In recent years, winemakers in Europe and other countries, such as Australia and South Africa, have shown an interest in using stems, and several technical articles mention the advantages of this practice [4,5].
These winemaking techniques are not used for all grape varieties, nor for all vintages. Since these techniques have very little research behind them, they are generally passed along by word of mouth. Therefore, it is difficult to know which stem conditions will lead to improved or deteriorated wine quality.
Stem composition has often been analyzed to value winemaking by-products and has been relatively well studied. Many compounds of interest can be found in stems’ overall composition. Their richness in polyphenolic compounds makes them very interesting for the food and medicine industries, in relation to their antioxidant potential. In some studies, units used to express stem extract composition is very specific and makes it impossible to compare results. Therefore, such data are not presented in this article [6,7,8,9,10]. This review gathered information from the literature on stem chemical composition to examine how these compounds contribute to variations in aroma and taste when stems are included during winemaking. Although stems are only used for red wines, we also examined data on the chemical composition of white grape variety stems. We then compiled the main results observed when whole clusters of grapes or single stems were incorporated into the winemaking process, from a technological, chemical, and sensorial perspective.
2. Grape Stems
2.1. Morphology
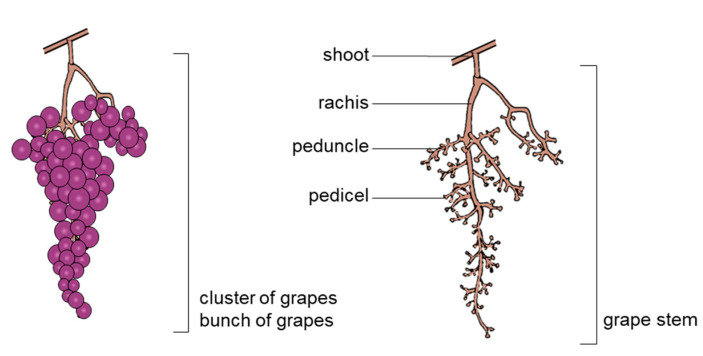
The stem is the skeleton of the grape cluster or bunch. The longest part, the rachis (main axis), is branched with peduncles, and a pedicel attaches each grape berry to the stem (Figure 1).
Figure 1.
Bunch of grapes and stem morphology.
The stem’s final size is reached around veraison [1]. For each grape variety, the number, length, and distance between two ramifications varies. Along with other morphological criteria, these components determine the compactness of the bunch [11]. The stem accounts for 3 to 7% of the total bunch weight, depending on the grape variety, number of grapes on the bunch, and its sanitary state [1,2,12].
2.2. General Composition
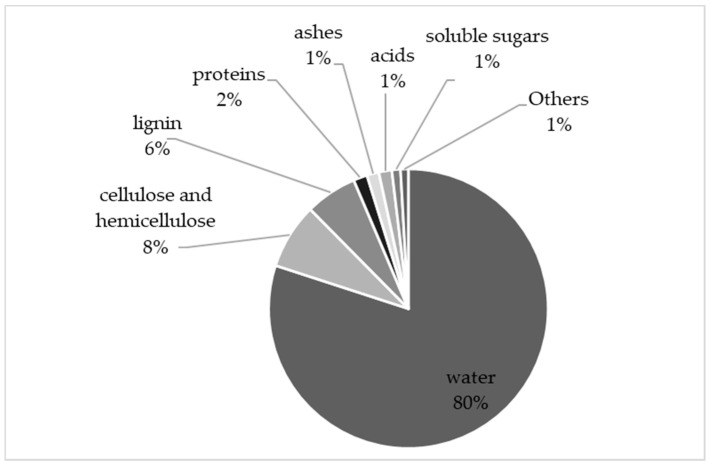
This part of the review aims to summarize the main compounds found in grape stems. An estimation of their quantification based on the available data is shown in Figure 2. This composition is close to the one described by Foulonneau et al. which is similar to that of the vine’s leaves and tendrils [2]. For each type of compound, available data were summarized. It should be noticed that the comparison of published data is difficult, as their proportions can be impacted by different factors, such as grape variety, vintage, maturation state, as well as differences in extraction techniques and units.
Figure 2.
General composition of grape stems.
2.2.1. Water
As the stem’s main component, water accounts for 55 to 80% of stem weight [2,3,13,14]. In 1976, Rice et al. measured the moisture of fresh grape stems from ten grape varieties, five reds (Concord, Ives, Baco noir, Red hybrids, and Cascade) and five whites (Aurore, Concord CP, Delaware, Niagara, and Catawba) planted in New York state, USA [14]. The values ranged between 68.4 and 79.1% of stem fresh weight (FW). No significant differences were found between red and white grapes and variability was imputed to the grape variety. In 2010, Gonzalez-Centeno et al. studied the overall stem composition of ten other grape varieties, six reds (Cabernet Sauvignon, Callet, Manto Negro, Merlot, Syrah, and Tempranillo) and four whites (Chardonnay, Macabeu, Parellada, and Premsal Blanc) planted on Mallorca Island, Spain, and found similar values, ranging from 55 to 80% of FW [13]. Of the grape varieties studied, white grape varieties appeared to have significantly higher moisture content (71.7 g/100 g FW) than red varieties (62.5 g/100 g FW). Stem water content appeared to depend on the grape variety. However, none of these studies considered stem maturity, which could have a major influence on the values.
2.2.2. Cellulose and Hemicellulose
In stems, as in classical vegetable biomass [15], cellulose is the most abundant biopolymer followed by hemicelluloses (mannans, xyloglucans, xylans) [13,16,17,18,19]. Cellulose content values range from 12 to 38% dry matter (DM) (Table 1). The observed large variability might relate to differences in analytical procedures (extraction, analyses, and calculation) [17] and/or variability between grape varieties [20].
Table 1.
Main chemical components of stems (values expressed in % DM).
| Grape Variety | Cellulose | Hemicellulose | Lignin | Proteins | Ash |
|---|---|---|---|---|---|
| Pinot Noir [17] (Bellucci method) | 24.65 | 7.66 | |||
| Pinot Noir [17] (Sluiter et al. method) | 25.3 | 13.95 | 47.29 | 7.66 | |
| Pinot Noir [17] (Goering-Van Soest method) | 37.88 | 14.93 | 32.98 | 7.66 | |
| Red grapes [19] | 34.6 | 14.5 | |||
| Vitis vinifera L. [18] | 30.3 | 21 | 17.4 | 6.1 | 7.0 |
| Cabernet Sauvignon [13] | 23.0 | 11.6 | 12.8 to 22.6 | 5.8 | 10.8 |
| Callet [13] | 23.3 | 13.1 | 8.3 | 7.1 | |
| Manto Negro [13] | 23.1 | 13.7 | 6.7 | 6.9 | |
| Merlot [13] | 27.1 | 12.7 | 5.7 | 11.2 | |
| Syrah [13] | 35.0 | 17.2 | 6.8 | 4.8 | |
| Tempranillo [13] | 19.6 | 10.2 | 4.9 | 10.0 | |
| Chardonnay [13] | 26.1 | 11.8 | 7.7 | 8.6 | |
| Macabeu [13] | 25.0 | 13.6 | 6.6 | 5.5 | |
| Parellada [13] | 26.3 | 14.0 | 11.2 | 6.4 | |
| Premsal Blanc [13] | 22.2 | 9.8 | 9.2 | 5.9 | |
| Manto Negro [23] | 31.6 | 7.29 | 5.48 | ||
| Premsal Blanc [21] | 22.91 | 5.12 | 6.94 | ||
| Alsacian white grape variety [16] | 36.3 | 24.5 | 39.6 | 3.9 | |
| Mix of Bonarda and Barbera [20] | 12.19 | 25.7 | 32.35 | 6.11 | |
| Albariňo [24] | 29.95 | 35.33 | 22.94 |
2.2.3. Lignin
Lignin content ranges from 13 to 47% of DM (Table 1), with many studies reporting on the variability and providing different explanations, such as analytical method [17,18], grape variety [16,18], or stem maturity [21]. Indeed, studies have used different measurement and calculation methods to evaluate the lignin content, with some including acid soluble and insoluble lignin [16,17] and others including only acid-insoluble residues as the amount of lignin [18]. These method variations can lead either to an over- or under-estimation of total lignin content. The stem’s ripening speed depends mainly on the grape variety and climatic conditions [22]. Full lignification often occurs beyond berry maturity [1]. Indeed, the maturity stage of the stem at harvest will affect its composition. To our knowledge, stem maturity has not been considered in previous studies. It would be interesting to evaluate this maturity to better understand stem composition evolution during maturation.
2.2.4. Proteins
Stem protein content ranges from 5 to 11% DM (2–3% of fresh weight) with a mean of 7% [13,18,21,23] (Table 1). The obtained values do not consider whether variations are related to grape variety or only to the biological variability of the raw material induced either by stem maturity or the extraction process (drying, crushing, etc.). These values are consistent because stems are not vine storage organs. Notably, different studies mention the presence of resistant proteins, referring to proteins bound with lignin, which are difficult to access, suggesting that the protein level could be underestimated [21,23]. Total protein quantification is, therefore, complex.
2.2.5. Ashes
As with protein content, reported ash content is relatively consistent across different studies, with a mean value of 6.9% DM, regardless of the grape variety or origin (Table 1). Prozil et al. used inductive coupled plasma (ICP) to analyze detailed metal cation composition and identified potassium as the main mineral element of grape stems (K: 0.9%, Ca: 0.15%, Mg: 0.02%, Zn: 0.01% and Na < 0.01% of total ash content) [18].
2.2.6. Acids
Stem acidic composition has been estimated by measuring stem extracts’ total acidity using a reaction with Bromothymol blue, with values ranging from 13.5 to 15.0 g/kg FW, or approximatively 1 to 2% of the total stem weight [2]. No information regarding further analysis of acid types was found in the literature.
2.2.7. Sugars
Stems have a low sugar content [2,21]. According to Gonzalez-Centeno et al. soluble sugar content, determined as glucose, according to the Haas colorimetric method (which uses anthrone as the reactive and measures the absorbance at 620 nm), ranges between 1.8 and 3.7 g/100 g stem FW [13]. Sugar concentration variability is related to grape variety rather than color. Similar values were found in other studies: 1.70% for Manto Negro [23], 1.04% for Premsal Blanc [21], with soluble sugar content lower than 10 g/kg of stem FW [1]. Therefore, stems do not represent a significant sugar input for fermentation compared to grape berries (sugar content 14.9 g/100 g FW) [13].
The main components of grape stems and their respective concentration, as described in the literature, are summarized in Table 1.
2.3. Polyphenolic Composition
Phenolic compounds are widely present in the plant kingdom, and red grape varieties contain a high concentration of these compounds, especially in grape solid parts, skin, and seeds. Studies have also reported their presence in vine-shoots [25,26,27]. Stem extract analysis found that stems are rich in polyphenolic compounds [28,29], with intermediate concentrations between the higher concentrations in grape seeds and the lower concentrations in grape skins [30].
2.3.1. Total Phenolic Content
The Folin–Ciocalteu method is a common technique for estimating total polyphenolic content in a vegetal fraction. Gallic acid is used as the standard and results are reported in gallic acid equivalent (GAE). Table 2 presents data from the literature on white and red grape varieties. To allow for comparison between the reported results, the unit was standardized (mg GAE/100 g DM). Grape stems show a wide range of total polyphenolic content. In white grape varieties, total polyphenolic values range from 400 to 22,900 mg GAE/100 g DM and in red grape varieties, similar values were found, ranging between 348 to 38,400 mg GAE/100 g DM. Variability in total polyphenol content can be attributed to many factors. Grape variety is one of these factors, as shows the important difference between the content measured in Asyrtiko (1248 and 1115 mg) and Athiri (400 and 480 mg) grape varieties by Anastasiadi et al. in two consecutive years [29], or in Chardonnay (4764 mg), and Pemsal blanc (9002 mg) by González-Centeno et al. [31]. Furthermore, Anastasiadi et al. and several other studies have also observed an important effect of vintage [29,32,33]. The importance of vineyard localization on the polyphenolic content was highlighted by the study of Spatafora et al. and Gouvinhas et al. demonstrated the effect of altitude [33,34].
Table 2.
Total polyphenol content of stems of white and red grape varieties (mg GAE/100g DM).
| Grape Variety | Vintage | Total Polyphenol Concentration |
|---|---|---|
| White Grape Varieties | ||
| Aidani [29] | 2009 | 1072.6 |
| Aidani [29] | 2010 | 722 |
| Asyrtiko [29] | 2009 | 1248 |
| Asyrtiko [29] | 2010 | 1114.6 |
| Athiri [29] | 2009 | 399.9 |
| Athiri [29] | 2010 | 480.8 |
| Chardonnay [31] | 2009 | 4764 |
| Chasselas [39] | 2015 | 300 to 4300 c |
| Fernao Pires [44] | 2017 | 11,015 |
| French Colombard [32] | 1987 | 2430 a |
| French Colombard [32] | 1988 | 1980 a |
| Macabeu [31] | 2009 | 7809 |
| Malvasia Fina [44] | 2017 | 12,309 |
| Moscatel (Sanfins du Douro) [33] | 2017 | 3235 |
| Moscatel (Sanfins du Douro) [33] | 2018 | 8305 |
| Moscatel (Penajóia) [33] | 2017 | 7802 |
| Moscatel (Penajóia) [33] | 2018 | 10,349 |
| Moscatel (Medrões) [33] | 2017 | 3793 |
| Moscatel (Medrões) [33] | 2018 | 8832 |
| Moscatel [44] | 2017 | 10,871 |
| Parellada [31] | 2009 | 8924 |
| Premsal blanc [31] | 2009 | 9002 |
| Premsal blanc [21] | - | 8730 |
| Premsal blanc [45] | - | 17,200 to 22,900 d |
| Rabigato [44] | 2017 | 9471 |
| Roditis [35] | - | 3120 to 7468 d |
| Semillon [32] | 1987 | 1950 a |
| Semillon [32] | 1988 | 1690 a |
| Viosinho [44] | 2017 | 9699 |
| Red Grape Varieties | ||
| Cabernet [39] | 2015 | 1200 to 2000 c |
| Cabernet Sauvignon [31] | 2009 | 7076 |
| Cabernet Sauvignon [28] | 2000 | 2500 b |
| Cabernet Sauvignon [34] | 2009 | 348.0 |
| Callet [31] | 2009 | 11,525 |
| Carnelian [32] | 1987 | 2170 a |
| Carnelian [32] | 1988 | 1850 a |
| Frappato [34] | 2009 | 998.5 |
| Mandilaria [29] | 2009 | 1057 |
| Mandilaria [29] | 2010 | 1434.3 |
| Manto Negro [31] | 2009 | 8470 |
| Manto Negro [23] | - | 11,600 |
| Manto Negro [45] | - | 29,400 to 38,400 d |
| Mavrotragano [29] | 2009 | 1011.1 |
| Mavrotragano [29] | 2010 | 557.9 |
| Mazuelo [38] | 2016 | 1276 to 5104 b,d |
| Merlot [31] | 2009 | 4704 |
| Merlot [39] | 2015 | 900 to 2900 c |
| Nerello Mascalese (Lingualossa) [34] | 2009 | 2179.8 |
| Nerello Mascalese (Milo) [34] | 2009 | 4000.1 |
| Nerello Mascalese (Santa Venerina) [34] | 2009 | 1241.7 |
| Nero d’Avola [34] | 2009 | 2632.9 |
| Ruby Cabernet [32] | 1987 | 1950 a |
| Ruby Cabernet [32] | 1988 | 1730 a |
| Sousao [46] | - | 3135 a |
| Syrah [31] | 2009 | 9642 |
| Syrah [28] | 2000 | 2500 to 5000 b,e |
| Syrah [39] | 2015 | 200 to 2250 c |
| Tempranillo [42] | - | 4679 |
| Tempranillo [31] | 2009 | 7622 |
| Tempranillo [28] | 2000 | 1250 to 3750 b,e |
| Voidomato [29] | 2009 | 840.2 |
| Voidomato [29] | 2010 | 610 |
a Unit mg GAE/100 g FM. b Unit mg GAE/L/100 g DM. c According to the size of the stem parts during extraction. d According to the extraction method. e According to the irrigation of the vine.
In addition to the effect related to the grape and its growing conditions, several authors highlighted the impact of the extraction method on total polyphenolic content in the same grape variety. Makris et al. obtained values ranging from 3120 to 7468 mg GAE/100 g DM for Roditis grape variety, merely by changing the composition of the extraction solution [35,36,37]. Jimenez-Moreno et al. obtained similar results on Mazuelo stems, with values ranging from 1276 to 51,045 mg GAE/L/100 g of DM. They highlighted the fact that ethanol concentration was the most determinant parameter among temperature, solid/solvent ratio and ethanol content [38]. Interactions between these three different extraction parameters were also found. The size of the stem particles used for extraction also had an impact on the total polyphenol content, where smaller particles increased the exchange surface, allowing better extraction [39].
Further investigation to develop a standardized extraction method could help to compare results, including factors linked to either the grapes (e.g., grape variety [31,32,37,40,41], vintage conditions [29], vineyard localization [31,42], stem maturity [43]) or to differing extraction parameters [36,37] (e.g., solvent, duration, stem particle size, temperature).
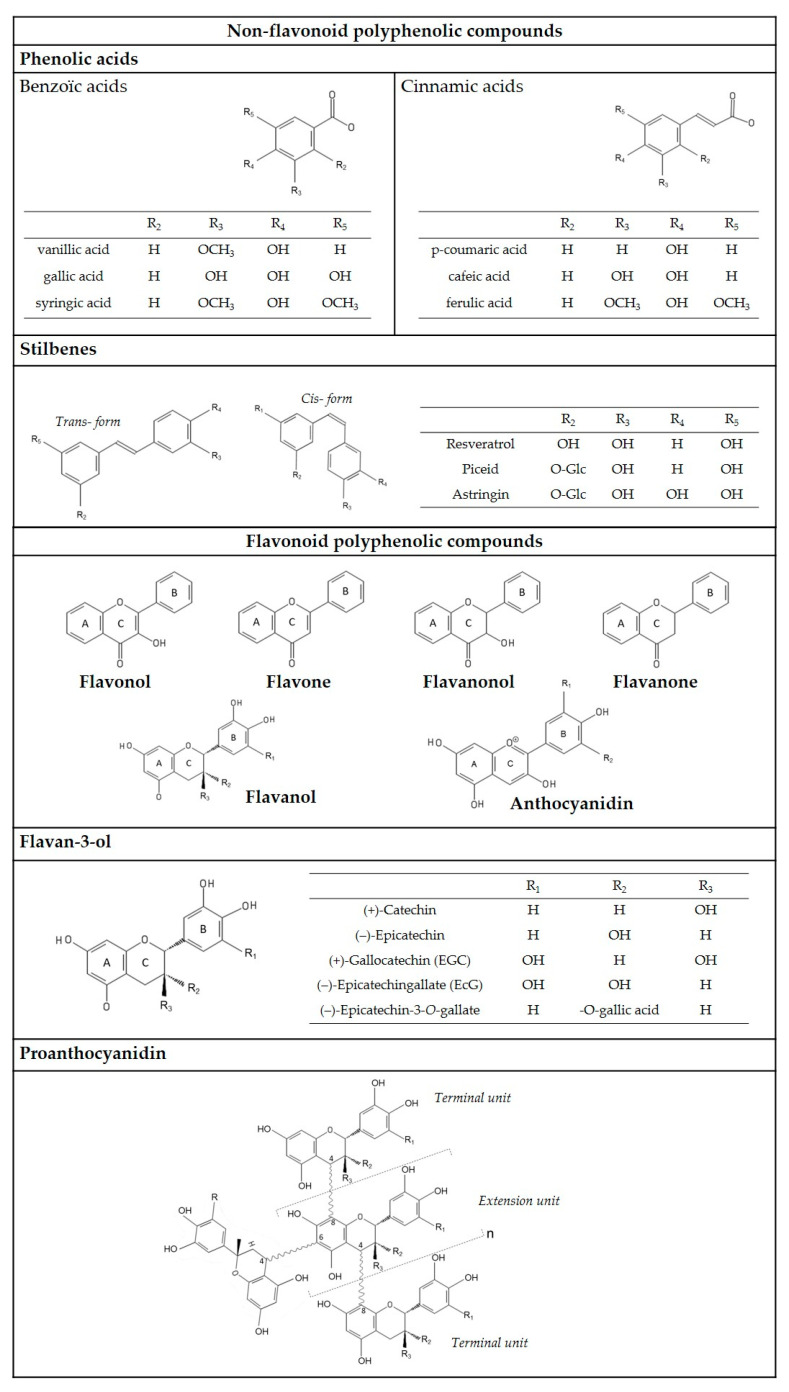
2.3.2. Non-Flavonoid Compounds
The total phenolic content comprises an important variety of molecules containing phenol rings in their chemical structure. Polyphenolic compounds are divided into two major categories, flavonoids and non-flavonoids. The presence of both types of molecules has been studied in grape stem extracts, with detailed molecular compositions reported in the literature.
Non-flavonoid molecules include phenolic acids, stilbenes, and hydrolysable tannins (Figure 3). To our knowledge, no hydrolysable tannin content has been reported in the literature.
Figure 3.
Non-flavonoid and flavonoid polyphenolic compound of grapes and wine.
Phenolic Acids
Different phenolic acids have been identified in grape stem extracts: caftaric acid [28,36,37,39,40,44,47,48], coutaric acid [28,40], gallic acid [19,28,29,34,38,39,48,49], coumaric acid [19,28,29,41], caffeic acid [29,41], syringic acid [19,28,29], ferulic acid [29], protocatechuic acid [48], trans-cinnamic acid [48], and other unidentified hydrocynnamic acids [44,47]. Many of these acids have also been identified in other grape parts, such as pulp, skin or seeds, or in other wine by-products, such as pomace [28,40,50].
Some authors have quantified these different phenolic acids using HPLC techniques. Results for white and red grape varieties are shown in Table 3. When the units differed, the values were standardized by converting to mg/kg DM. Comparing these values across studies remains difficult because extraction protocols differed according to the study and not all papers quantified phenolic acids. However, the major phenolic acids in stems appear to be caftaric acid and gallic acid. Caftaric acid was found in a concentration between 5.1–12,820 mg/kg DM in white grape varieties and 12.5–1500 mg/kg DM in red varieties. Gallic acid reached concentrations of 30–469 mg/kg DM in white varieties and 6.5–300 mg/kg DM in red varieties. As for the total phenolic content, individual phenolic acid concentrations are influenced by the grape variety [28,29,44], geographical origin [34], and the vintage [29]. The study of Gouvinhas et al. shows that the concentration of phenolic acids, such as caftaric and hydroxycinnamic acids, are highly correlated to the altitude and the vintage [33]. A strong thermal and water stress, related to the lower altitude, increases the synthesis of phenolic compounds in the plant and consequently in the stem. The effect of water stress is well demonstrated in the study of Alonso et al. where, in the no irrigated variants of Tempranillo and Syrah stems, the concentration of major phenolic acids, namely caftaric and coutaric acids, are significantly higher than in the irrigated variants [28]. In wine, these compounds have no odor, nor aroma. However, they can be precursors to volatile phenols, molecules that can induce defects in wines. Their extraction from stems is not necessarily interesting for winemaking.
Table 3.
Quantification of phenolic acids in white and red grape varieties (mg/kg DM).
| Grape Variety | Vintage | Caftaric Acid | Coutaric Acid | Coumaric Acid | Gallic Acid | Caffeic Acid | Syringic Acid | Ferulic Acid |
|---|---|---|---|---|---|---|---|---|
| White Grape Varieties | ||||||||
| Aidani [29] | 2009 | 71.6 | 0.9 | 171 | 1.4 | 3.5 | 1.1 | |
| Aidani [29] | 2010 | 136 | 0.1 | 105 | 0.1 | n.d. | n.d. | |
| Arinto [48] | 2018 | 220 | 40 | |||||
| Asyrtiko [29] | 2009 | 69.6 | 1.1 | 469 | n.d. | 1.8 | n.d. | |
| Asyrtiko [29] | 2010 | 146 | 0.7 | 454 | 0.4 | n.d. | n.d. | |
| Athiri [29] | 2009 | 5.1 | 0.6 | 122 | 0.5 | 0.4 | n.d. | |
| Athiri [29] | 2010 | 6.1 | 0.7 | 146 | 0.5 | 0.5 | 0.2 | |
| Chasselas [39] | 2015 | 1500 to 3600 b | 30 to 250 b | |||||
| Fernao Pires [47] | - | 100 | ||||||
| Fernao Pires [44] | 2017 | 1710 | ||||||
| Fernao Pires [48] | 2018 | 680 | 50 | |||||
| Malvasia Fina [44] | 2017 | 12,820 | ||||||
| Moscatel (Sanfins du Douro) [33] | 2017 | 135 | ||||||
| Moscatel (Sanfins du Douro) [33] | 2018 | 342 | ||||||
| Moscatel (Penajóia) [33] | 2017 | 480 | ||||||
| Moscatel (Penajóia) [33] | 2018 | 485 | ||||||
| Moscatel (Medrões) [33] | 2017 | 203 | ||||||
| Moscatel (Medrões) [33] | 2018 | 338 | ||||||
| Moscatel [44] | 2017 | 5010 | ||||||
| Rabigato [47] | - | 250 | ||||||
| Rabigato [44] | 2017 | 2280 | ||||||
| Viosinho [47] | - | 200 | ||||||
| Viosinho [44] | 2017 | 1510 | ||||||
| Red Grape Varieties | ||||||||
| Amarela [47] | - | 300 | ||||||
| Cabernet Sauvignon (irrigated) [28] | 2000 | 20.3 | 11.7 | 8.1 | 6.9 | 14.7 | ||
| Cabernet Sauvignon (non irrigated) [28] | 2000 | 30.8 | 2.7 | 1.4 | n.d. | 11.4 | ||
| Cabernet Sauvignon [39] | 2015 | 900 to 1500 b | 80 to 260 b | |||||
| Castelao [48] | 2018 | 200 | n.d. | |||||
| Mandilaria [29] | 2009 | 57.9 | 0.9 | 286 | 5.3 | 1.4 | 0.7 | |
| Mandilaria [29] | 2010 | 41.1 | 1.5 | 70.4 | 1.3 | n.d. | 0.6 | |
| Mavrotragano [29] | 2009 | 166 | 4 | 182 | 9.2 | 1.2 | 1.5 | |
| Mavrotragano [29] | 2010 | 78.4 | 1.1 | 90 | 2.8 | 0.3 | n.d. | |
| Mazuelo [38] | 2016 | 43 to 310 c | ||||||
| Merlot [40] | - | 40 a | 4.5 a | |||||
| Merlot [39] | 2015 | 800 to 1400 b | 80 to 300 b | |||||
| Nerello Mascalese (Milo) [34] | 2009 | 87.2 | ||||||
| Nerello Mascalese (Lingualossa) [34] | 2009 | 71.9 | ||||||
| Nero d’Avola [34] | 2009 | 49.8 | ||||||
| Sousao [47] | - | 900 | ||||||
| Syrah (irrigated) [28] | 2000 | 12.5 | n.d. | n.d. | 7.6 | n.d. | ||
| Syrah (non irrigated) [28] | 2000 | 95.6 | 38 | 9 | n.d. | n.d. | ||
| Syrah [39] | 2015 | 200 to 1600 b | 10 to 150 b | |||||
| Syrah [48] | 2018 | 400 | 40 | |||||
| Tempranillo (irrigated) [28] | 2000 | 31.5 | 16.3 | n.d. | n.d. | n.d. | ||
| Tempranillo (non irrigated) [28] | 2000 | 66.9 | 32.8 | 0.9 | n.d. | n.d. | ||
| Tinta Barroca [47] | - | 1100 | ||||||
| Tinta Roriz [48] | 2018 | 430 | n.d. | |||||
| Touriga Nacional [47] | - | 500 | ||||||
| Touriga Nacional [48] | 2018 | 980 | n.d. | |||||
| Voidomato [29] | 2009 | 274 | 2 | 195 | 8.6 | 2.4 | n.d. | |
| Voidomato [29] | 2010 | 53.9 | 0.7 | 278 | 3.5 | 1.4 | n.d. | |
a Unit mg/kg FM. b According to the size of the stem parts during extraction. c According to the extraction method.
Stilbenes
Trans-resveratrol and ε-viniferin are the two main stilbenes identified in grape stem extracts [29,33,34,36,38,41,44,47,48,49,51]. Values found in the literature are summarized in Table 4. Trans-resveratrol values ranged from 31 to 393 mg/kg DM and ε-viniferin, value range from 1.91 to 900 mg/kg DM. According to several authors, the differences between the values are mainly due to the different cultivars, geographical regions, and vintages [29,33,52]. As mentioned in Piñiero et al.’s study, the extraction protocol has a great impact on the extraction yields, especially the ethanol concentration and the sample-solvent ratio [52]. On the other hand, extraction duration (between 15 and 35 min) did not have a significant impact. Bavaresco et al. studied the transfer of stilbenoid compounds in wine in extraction conditions similar to wine (11% (v/v) ethanol and 250 ppm (v/v) methanol) and found only trans-resveratrol in the extract. The values ranged from 6.0 to 17.8 mg/kg DM, meaning that the level of stilbenes potentially extractable during maceration would be lower than the values found in the extracts presented in Table 4. The presence of other stilbenoid compounds, such as piceatannol, was also mentioned in the literature [41,52]. Finally, some studies reported that no stilbenes were found in the stem extracts [37,44]. This could either be related to the extraction method or to grape stem composition variability.
Table 4.
Quantification of trans-resveratrol and ε-viniferin in white and red grape varieties (mg/kg DM).
| Grape Variety | Vintage | trans-Resveratrol | ε-Viniferin |
|---|---|---|---|
| White Grape Varieties | |||
| Aidani [29] | 2009 | 74 | 167 |
| Aidani [29] | 2010 | 124 | 174 |
| Arinto [48] | 2018 | 80 | 70 |
| Asyrtiko [29] | 2010 | 178 | 253 |
| Asyrtiko [29] | 2009 | 87.6 | 223 |
| Athiri [29] | 2009 | 96 | 415 |
| Athiri [29] | 2010 | 115 | 499 |
| Castealo [48] | 2018 | 70 | 70 |
| Chardonnay [52] | 2010 | n.d. | 60.6 |
| Chardonnay [52] | 2012 | 42.2 | 25.7 |
| Fernao Pires [53] | 2013 | 1.91 | |
| Fernao Pires [44] | 2017 | 900 | |
| Fernao Pires [48] | 2018 | 90 | 80 |
| Gewürztraminer [51] | - | 393 a | 69 a |
| Malvasia Fina [44] | 2017 | 170 | |
| Moscatel [44] | 2017 | 760 | |
| Moscatel (Medrões) [33] | 2017 | 97 | |
| Moscatel (Medrões) [33] | 2018 | 122 | |
| Moscatel (Penajóia) [33] | 2017 | 75 | |
| Moscatel (Penajóia) [33] | 2018 | 96 | |
| Moscatel (Sanfins du Douro) [33] | 2017 | 23 | |
| Moscatel (Sanfins du Douro) [33] | 2018 | 39 | |
| Moscato [51] | - | 100 a | 56 a |
| Palomino fino [52] | 2010 | traces | 24.7 |
| Palomino fino [52] | 2012 | n.d. | 14.3 |
| Pinot Gris [51] | - | 159 a | 34 a |
| Rabigato [53] | 2013 | 29.9 | |
| Rabigato [44] | 2017 | 310 | |
| Sauvignon [51] | - | 95 a | 171 a |
| Sauvignon blanc [52] | 2010 | n.d. | 147.1 |
| Tocai friulano [51] | - | 82 a | 35 a |
| Vijiriega [52] | 2010 | traces | 48 |
| Viosinho [53] | 2013 | 26.1 | |
| Viosinho [44] | 2017 | 830 | |
| Red Grape Varieties | |||
| Cabernet franc [51] | - | 238 a | 138 a |
| Cabernet Sauvignon [52] | 2012 | n.d. | 17.6 |
| Granacha [52] | 2010 | traces | 29.4 |
| Mandilaria [29] | 2009 | 266 | 476 |
| Mandilaria [29] | 2010 | 176 | 282 |
| Marzemino [51] | - | 31 a | n.d. |
| Mavrotragano [29] | 2009 | 87.6 | 258 |
| Mavrotragano [29] | 2010 | 96.5 | 235 |
| Mazuelo [38] | 2016 | 21 to 162 b | 91 to 310 b |
| Merlot [51] | - | 38 a | 54 a |
| Merlot [52] | 2012 | n.d. | 30.1 |
| Nerello Mascalese (Lingualossa) [34] | 2009 | 158.85 | 176.13 |
| Nerello Mascalese (Milo) [34] | 2009 | 102.63 | 114.75 |
| Nero d’Avola [34] | 2009 | 111.07 | 25.80 |
| Petit Verdot [52] | 2012 | n.d. | 20.5 |
| Sousao [53] | 2013 | 24.8 | |
| Syrah [48] | 2018 | 60 | 70 |
| Syrah [52] | 2010 | 122.5 | 71.1 |
| Syrah (treatment A) [52] | 2010 | 135.4 | 52 |
| Syrah (treatment A) [52] | 2011 | 64 | 41.7 |
| Syrah (treatment B) [52] | 2011 | 139.1 | 65.1 |
| Tempranillo 1 [52] | 2010 | 79.8 | 60.5 |
| Tempranillo 2 [52] | 2010 | 87.8 | 80.6 |
| Tempranillo [52] | 2012 | n.d. | 28.3 |
| Tinta Amarela [53] | 2013 | 2.2 | |
| Tinta Baroca [53] | 2013 | 10.8 | |
| Tinta Roriz [48] | 2018 | 70 | 70 |
| Tintilla de Rota [52] | 2010 | 118.9 | 91.6 |
| Tintilla de Rota [52] | 2012 | traces | 39.2 |
| Touriga Nacional [53] | 2013 | 11.4 | |
| Touriga Nacional [48] | 2018 | 140.00 | 110 |
| Vitis silvestris 1 [52] | 2010 | 49.9 | 59 |
| Vitis silvestris 2 [52] | 2010 | traces | 38.7 |
| Vitis silvestris 3 [52] | 2010 | 33 | 74.8 |
| Voidomato [29] | 2010 | 174 | 414 |
| Voidomato [29] | 2009 | 92.9 | 217 |
a Unit mg/kg FM. b According to the extraction method.
2.3.3. Flavonoid Compounds
Flavonoid compounds share the same basic structure formed by two aromatic rings linked by three carbons: C6-C3-C6. This group of molecules includes flavonols, flavanols, flavanonols, flavones, flavanones (intense yellow pigments), and anthocyanins (red or blue pigments). Flavan-3-ols form oligomers and polymers, called proanthocyanidins or condensed tannins. Their different structures are presented in Figure 3. The most common flavonoid compounds in grapes and wines are flavonols, flavanols, anthocyanidins, and their derivatives.
Only one recent study tentatively identified a flavone in stem extracts, chrysoeriol malonyl-apiosyl-glucoside [36]. To our knowledge, no other flavones or flavanones content has been reported in the literature.
Flavonols
The different flavonols identified in grape stem extracts are quercetin 3-O-glucuronide [34,36,37,40,44,47,49], quercetin 3-O-glucoside [29,34,40,41], kaempferol 3-O-glucoside [36,40,44,47], myricetin 3-O-glucoside [40], myricetin 3-O-glucuronide [40], quercetin 3-O-rutinoside [37,39,44,47,48,49], quercetin 3-O-galactoside [29], quercetin 3-O-rhamnoside [29], kaempferol [29], quercetin [29], isorhamnetin-3-O-(6-O-feruloyl)-glucoside [44,47], and kaempferol-3-O-rutinoside [44,47].
Different authors have reported concentration values of these compounds for various white and red varieties (Table 5). Quercetin derivatives were reported to be the main flavonols followed by kaempferol derivatives. Quercetin-3-O-glucuronide, quercetin-3-O-rutinoside and quercetin-3-O-galactoside appeared to be the most abundant flavonols in grape stem extracts, depending on the extraction solvent used for sample preparation. The solubility in water of flavonol derivatives increases in the following order: rhamnoside < glucoside < galactoside < glucuronide < rutinoside [54]. Using only water for the extraction Kosinska–Cagnazzo et al. found only quercetin-3-O-rutinoside in the extracts, and the quantity varies with the size of the stems [39] when Barros et al. and Leal et al. with 50 and 70% of methanol in water extracted meanly quercetin-3-O-glucuronide [44,47]. The addition of organic solvent allows for the extraction of more apolares substances, such as kaempferol derivatives. However the high amount of organic compounds in the extraction mixture, as the 90% acetonitrile used by Anastasiadi et al. could conduct to the loss of the water-soluble derivatives [29]. This demonstrates the importance of the extraction conditions on the profile and the quantity of polyphenols measured in stems.
Table 5.
Flavonol content quantified in different grape stem extracts (mg/kg DM).
| Grape Variety | Vintage | Quercetin-3-O-Galactoside | Quercetin-3-O-Glucoside | Quercetin-3-O-Rhamnoside | Quercetin-3-O-Glucuronide | Quercetin-3-O-Rutinoside | Quercetin | Kaempferol | Kaempferol-3-O-Rutinoside | Kaempferol 3-O-Glucoside |
|---|---|---|---|---|---|---|---|---|---|---|
| White Grape Varieties | ||||||||||
| Aidani [29] | 2009 | 87.2 | 57.7 | 17.3 | 9.4 | 0.5 | ||||
| Aidani [29] | 2010 | 197 | 71.5 | 19.3 | 7.3 | 1 | ||||
| Arinto [48] | 2018 | 150 | ||||||||
| Asyrtiko [29] | 2009 | 193 | 65.1 | 4.6 | 21 | 1.3 | ||||
| Asyrtiko [29] | 2010 | 305 | 137 | 24.1 | 5.6 | 0.8 | ||||
| Athiri [29] | 2009 | 142 | 50.9 | 15.8 | 7.7 | 1.4 | ||||
| Athiri [29] | 2010 | 170 | 61.1 | 19 | 9.2 | 1.6 | ||||
| Chasselas [39] | 2015 | 600 to 3000 b | ||||||||
| Fernao Pires [47] | - | 400 | 70.0 | 60 | 50 | |||||
| Fernao Pires [44] | 2017 | 40,270 | 140 | 140 | 40 | |||||
| Fernao Pires [48] | 2018 | 440 | ||||||||
| Malvasia Fina [47] | 2017 | 73,790 | 190 | 400 | 20 | |||||
| Moscatel (Sanfins du Douro) [33] | 2017 | 211 | 22 | 5 | 19 | |||||
| Moscatel (Sanfins du Douro) [33] | 2018 | 285 | 117 | 8 | 21 | |||||
| Moscatel (Penajóia) [33] | 2017 | 387 | 45 | 10 | 29 | |||||
| Moscatel (Penajóia) [33] | 2018 | 445 | 187 | 12 | 30 | |||||
| Moscatel (Medrões) [33] | 2017 | 374 | 36 | 7 | 27 | |||||
| Moscatel (Medrões) [33] | 2018 | 423 | 104 | 10 | 29 | |||||
| Moscatel [44] | 2017 | 29,270 | 230 | 140 | 40 | |||||
| Viosinho [44] | 2017 | 34,630 | 50 | 100 | 40 | |||||
| Viosinho [47] | 800 | 80.0 | 30 | 75 | ||||||
| Red Grape Varieties | ||||||||||
| Amarela [47] | - | 600 | 50.0 | 55 | 33 | |||||
| Cabernet Sauvignon [39] | 2015 | 500 to 800 b | ||||||||
| Castelao [48] | 2018 | 240 | ||||||||
| Mandilaria [29] | 2009 | 127 | 54.1 | 6.7 | 12.7 | 4.4 | ||||
| Mandilaria [29] | 2010 | 243 | 130 | 4.2 | 10.3 | 0.7 | ||||
| Mavrotragano [29] | 2009 | 223 | 86.5 | 17.5 | 2 | 0.7 | ||||
| Mavrotragano [29] | 2010 | 149 | 70.1 | 8.4 | 9.5 | 1.8 | ||||
| Mazuelo [38] | 2016 | 96 to 485 c | 8 to 38 c | |||||||
| Merlot [39] | 2015 | 200 to 1000 b | ||||||||
| Merlot [40] | - | 18.0 a | 200.0 a | traces a | ||||||
| Nerello Mascalese (Milo) [34] | 2009 | 36.4 | 70.7 | |||||||
| Nerello Mascalese (Lingualossa) [34] | 2009 | 152.9 | 229.5 | |||||||
| Nero d’Avola [34] | 2009 | 65.7 | 161.3 | |||||||
| Rabigato [47] | - | 350.0 | 50.0 | 25 | 25 | |||||
| Rabigato [44] | 2017 | 37,560 | 150 | 40 | 20 | |||||
| Sousao [47] | - | 1380 | 120.0 | 75 | 25 | |||||
| Syrah [39] | 2015 | 50 to 600 b | ||||||||
| Syrah [48] | 2018 | 410 | ||||||||
| Tinta Barroca [47] | 140 | 120 | 150 | 30 | ||||||
| Tinta Roriz [48] | 2018 | 370 | ||||||||
| Touriga Nacional [47] | 700 | 25 | 80 | 20 | ||||||
| Touriga Nacional [48] | 2018 | 440 | ||||||||
| Voidomato [29] | 2009 | 205 | 65.5 | 15.3 | 13.7 | n.d. | ||||
| Voidomato [29] | 2010 | 126 | 61.4 | 23.8 | 19.6 | 2.3 | ||||
a Unit mg/kg FM. b According to the size of the stem parts during extraction. c According to the extraction method.
Flavanonols
Astilbin [36,37,40,49] and engeletin [40] are the two main flavanonols identified in grape stem extracts. Only Souquet et al. quantified astilbin (35 mg/kg of stems) and found traces of engeletin in the stem extracts [40]. Dihydroquercetin, also called taxifolin, is the flavanonol mainly identified in grapes and wine, and was not found in grape stem extracts.
Flavan-3-ols and Proanthocyanidins
The profile of flavan-3-ols and proanthocyanidins was measured in the stem extracts using HPLC-DAD or HPLC-MS techniques. Information about molecular ion and the typical fragments are summarized in Table 6.
Table 6.
Identification of flavan-3-ols and proanthocyanidins in grape stem extracts (ESI).
| Compound | [M-H]− (m/z) | [M-H]+ (m/z) | MS2 (m/z) |
|---|---|---|---|
| (+)-catechin [41] | 289 | 245; 205; 179; 203; 227; 165; 161 | |
| (+)-catechin [37,41] | 291 | 123; 139; 165; 273; 151; 147; 249 | |
| (−)-epicatechin [29,40,41,47,55] | 291 | 245; 205; 179; 203; 231; 271; 161 | |
| (−)-epicatechin [41] | 289 | 123; 139; 165; 151; 273; 147; 231 | |
| (epi)catechin gallate [29,47,55] | 441 | 331; 289; 169 | |
| Catechin gallate [41] | 441 | 289; 395; 169; 331; 245; 193; 405 | |
| Procyanidin dimer A [47] | 575 | 573; 477; 441 | |
| B1 Ec-(4β→8)-Cat [41] | 577 | 425; 407; 289; 451; 245; 287 | |
| B1 Ec-(4β→8)-Cat [41] | 579 | 427; 409; 291; 301; 247; 289; 287 | |
| B2 Ec-(4β→8)-Ec [41] | 577 | 425; 407; 287; 289; 451; 559; 299 | |
| Procyanidin dimer B [47] | 577 | 559; 425; 407; 287 | |
| Procyanidin dimer [36] | 577 | 289; 425; 407; 451; 559 | |
| (epi)gallocatechin-(epi)catechin dimer [47] | 593 | 575; 531; 425; 423; 273 | |
| Galloylated flavanol dimer (epi)catechin-(epi)catechin gallate [37,49] | 731 | 579; 291; 139 | |
| Procyanidin dimer gallate | 729 | 711; 577; 559; 451; 407; 289 | |
| Procyanidin dimer gallate [36] | 729 | 577; 559; 451; 407; 425; 289 | |
| Prodelphinidine gallate [36] | 745 | 593; 405; 575 | |
| Flavanol dimer [37] | 579 | 601 | |
| Flavanol trimer [49] | 867 | 579; 427 | |
| Procyanidin trimer [36] | 865 | 695; 577; 739; 451 | |
| Prodelphinidin trimer [36] | 881 | 695; 577; 755; 407; 303 | |
| Procyanidin trimer Gallate [36] | 1017 | 729; 407 | |
| Procyanidin tetramer [36] | 1153 | 865 | |
| Procyanidin tetramer [36] | 1169 | 881; 999; 1043; 729 | |
| Procyanidin pentamer [M-H]2− [36] | 720 | 635; 577; 521; 407 |
Among flavanols monomers, many studies reported the presence of catechin and epicatechin in grape stem extracts. Epicatechin gallate was found in two studies [29,47]. To our knowledge, no epigallocatechin was identified in grape stem extracts as a monomer unit.
Proanthocyanidin dimers and trimers were identified in stem extracts using the HPLC-MS technique: dimers B1, B2, B3, B4, B1-3-O-gallate, B2-3-O-gallate, B3-3-O-gallate, and trimers T2 and C1.
The three main compounds found in the stem extracts are catechin and the dimers B1 and B3 (Table 7). The proportion of all compounds seems to depend on the grape variety and the vintage, but also on the study. The choice of extraction conditions and analytical method essentially influences the profile reported by the different authors. This can be the reason why Alonso et al. did not find catechin in all extracts [28], when others found catechin content ranging from 50 to 7640 mg/kg DM. The epicatechin content is low compared to catechin content. Barros et al. reported the sum of catechin and epicatechin in the extracts, with values ranging from 22 to 32 mg/g DM, depending on the grape variety [47]. In general, the concentrations of dimers B1 and B3 were found in the same magnitude as catechin, from 133 to 1958 mg/kg DM, and from 41 to 993 mg/kg DM, respectively.
Table 7.
Quantification of different flavan-3-ols and proanthocyanidins in grape stem extracts.
| Grape Variety | Vintage | Cat | Ec | EcG | B1 | B2 | B3 | B4 |
|---|---|---|---|---|---|---|---|---|
| Unit: mg/g FM | ||||||||
| Castelao Frances [56] | 1998 | 1.3 | 0.7 | 3.5 | 0.4 | 0.2 | 1 | |
| Merlot [40] | - | 60 | traces | |||||
| Tinta Miuda [57] | 1996 | 64.4 | 2.2 | 128.2 | 3.4 | 27.1 | 3.1 | |
| Touriga Francesa [56] | 1998 | 2 | 0.5 | 5.8 | 1.2 | 1.2 | 0.4 | |
| Viosinho [56] | 1998 | 1.5 | 0.6 | 1.2 | 0.4 | 0.1 | 0.1 | |
| Unit: mg/kg DM | ||||||||
| Aidani [29] | 2009 | 699 | 51.6 | 77.0 | 48.8 | 383 | ||
| Aidani [29] | 2010 | 737 | 58 | 34.2 | 36 | 215 | ||
| Arinto [48] | 2018 | 660 | 30 | |||||
| Asyrtiko [29] | 2009 | 1089 | 18.2 | 59.1 | 36.2 | 454 | ||
| Asyrtiko [29] | 2010 | 1858 | 27.9 | 86.0 | 165 | 646 | ||
| Athiri [29] | 2009 | 385 | n.d. | 53.9 | 55.2 | 161 | ||
| Athiri [29] | 2010 | 462 | 12.3 | 64.7 | 66.2 | 193 | ||
| Cabernet Sauvignon [39] | 2015 | 500 to 800 | ||||||
| Cabernet Sauvignon [31] | 2009 | 493 | 31 | 564 | 21 | 120 | n.d. | |
| Cabernet Sauvignon (irrigated) [28] | 2000 | n.d. | 7.6 | |||||
| Cabernet Sauvignon (non-irrigated) [28] | 2000 | 368.8 | n.d. | |||||
| Callet [31] | 2009 | 453 | 16 | 454 | 20 | 156 | n.d. | |
| Castelao [48] | 2018 | 440 | 40 | |||||
| Chardonnay [31] | 2009 | 314 | 12 | 255 | 15 | 56 | n.d. | |
| Chasselas [39] | 2015 | 600 to 3000 | ||||||
| Fernao Pires [48] | 2018 | 1270 | 170 | |||||
| Macabeu [31] | 2009 | 93 | 0.5 | 133 | 11 | 45 | n.d. | |
| Mandilaria [29] | 2009 | 1261 | 70.9 | 108.0 | 96.6 | 482 | ||
| Mandilaria [29] | 2010 | 1691 | 94.6 | 71.3 | 46.2 | 993 | ||
| Manto Negro [31] | 2009 | 122 | 06 | 246 | 11 | 41 | n.d. | |
| Mavrotragano [29] | 2009 | 1077 | 79.8 | 130.0 | 108 | 587 | ||
| Mavrotragano [29] | 2010 | 1027 | 64.4 | 88.0 | 44.3 | 243 | ||
| Mazuelo [38] | 2016 | 225 to 710 | ||||||
| Merlot [31] | 2009 | 575 | 24 | 868 | 22 | 132 | n.d. | |
| Merlot [39] | 2015 | 200 to 1000 | ||||||
| Nerello Mascalese (Milo) [34] | 2009 | 3611.0 | 1370.2 | |||||
| Nerello Mascalese (Lingualossa) [34] | 2009 | 2066.3 | 793.3 | |||||
| Nero d’Avola [34] | 2009 | 1562.7 | 1771.4 | |||||
| Parellada [31] | 2009 | 1339 | 58 | 1877 | 48 | 222 | n.d. | |
| Premsal blanc [31] | 2009 | 740 | 40 | 1218 | 40 | 104 | n.d. | |
| Syrah [31] | 2009 | 1146 | 24 | 1320 | traces | 208 | ||
| Syrah [39] | 2015 | 50 to 600 | ||||||
| Syrah [48] | 2018 | 1330 | 110 | |||||
| Syrah (irrigated) [28] | 2000 | n.d. | 24.1 | |||||
| Syrah (non-irrigated) [28] | 2000 | n.d. | n.d. | |||||
| Tempranillo [31] | 2009 | 1269 | 111 | 1958 | 94 | 232 | n.d. | |
| Tempranillo [42] | - | 7640 | ||||||
| Tempranillo (irrigated) [28] | 2000 | 674.4 | 338.5 | |||||
| Tempranillo (non-irrigated) [28] | 2000 | 280.8 | n.d. | |||||
| Tinta Roriz [48] | 2018 | 1620 | 90 | |||||
| Touriga Nacional [48] | 2018 | 2030 | 180 | |||||
| Voidomato [29] | 2009 | 795 | 189 | 95.3 | n.d. | 349 | ||
| Voidomato [29] | 2010 | 712 | n.d. | 64.9 | n.d. | 138 | ||
Cat = catechin; Ec = epicatechin; EcG = epicatechin gallate; B1, 2, 3 and 4 = proanthocyanidins dimers.
Procyanidin B1 has been reported as the main oligomer in skins [58,59,60], whereas procyanidin B2 [60,61,62] is the main oligomer in seeds. Therefore, the phenolic composition of stem extracts is likely to be closer to grape skins.
Proanthocyanidins or condensed tannins are present in plants in different degrees of polymerization. When this degree is higher than three, these compounds cannot be quantified by actual HPLC-MS methods. Total proanthocyanidin content can be estimated by several methods. The Bate–Smith reaction is most commonly used and is based on the ability of condensed tannins to be depolymerized under acidic conditions. This chemical depolymerization, followed by auto-oxidation, generates anthocyanidins, hence they are also called “proanthocyanidins” [63]. The concentration of the resulting colored molecules can be measured by spectrophotometry to estimate the quantity of monomers included in the condensed tannins. Other techniques use a reaction between the nucleophile site of the tannin and an aldehyde, such as vanillin or DMACA, to produce a colored product where the measured intensity increases with the quantity of tannins, but decreases with the polymerization degree of the tannins, as only the terminal monomer is reactive. The DMACA method is based on the reaction between catechin and 4-dimethylaminocinnamaldehyde, resulting in the formation of a blue complex that absorbs red light (around 640nm). In the vanillin assay, vanillin is protonated in an acidic solution and reacts specifically with the flavan-3-ols, dihydrochalcones, and proanthocyanidins, producing a red-colored compound where the concentration is measured by spectrophotometry at a wavelength between 500 and 550 nm. In this case, catechin is often used as a standard. Methylcellulose precipitation method allows proanthocyanidic polymers to be selectively precipitated with methylcellulose (MC), with which they form insoluble complexes. The MC plays the same role here as the salivary proteins in tasting. Ammonium sulfate (NH4)2SO4 in the reaction medium increases its polarity, thus promoting complex insolubilization and precipitation. For the protein precipitation method, a known amount of protein (BSA) binds to the tannin in the sample, forming a protein–tannin complex that precipitates. Then, precipitate is washed by a ferric chloride solution, which forms a colored complex, the absorbance of which can be read on a spectrophotometer at 510 nm. The amount of color is proportional to the amount of tannins in the stem extract [64].
Values obtained for different grape varieties are presented in Table 8, classified according to the analysis method. As expected, different methods produced sensibly different results. Values are variable, even using the same analysis method, and these differences could not be linked to the grape color. Moreover, for the same grape variety (Premsal blanc), values found in two different studies were significantly different: 79.0 mg/g DM in Llobera et al. and 181.4 mg/g DM in Gonzalez-Centeno et al. [21,31]. Makris et al. showed that the extraction method can modify the measured total proanthocyanidin value for the same grape variety by a factor of 5 [35].
Table 8.
Total proanthocyanidin content of grape stem extracts (mg/g DM).
| Grape Variety | Total Proanthocyanidin |
|---|---|
| Method: Bate-Smith Reaction | |
| Cabernet Sauvignon [31] | 124.9 |
| Callet [31] | 202.3 |
| Chardonnay [31] | 79.1 |
| Macabeu [31] | 108.8 |
| Manto Negro [31] | 165.3 |
| Merlot [31] | 84.0 |
| Parellada [31] | 165.2 |
| Premsal blanc [31] | 181.4 |
| Syrah [31] | 161.4 |
| Tempranillo [31] | 147.3 |
| Manto Negro [23] | 103 |
| Premsal blanc [21] | 79.0 |
| Roditis [35] | 55.5 to 255.7 b,d |
| Method: LCMS/MS Quantification | |
| Amarela [47] | 39 |
| Fernao Pires [47] | 35 |
| Rabigato [47] | 27 |
| Sousao [47] | 45 |
| Tinta Barroca [47] | 45 |
| Touriga Nacional [47] | 37 |
| Viosinho [47] | 27 |
| Method: Vanillin Assay | |
| Castelao Frances [56] | 53.7 a |
| Manto Negro [45] | between 217 and 270 c |
| Premsal blanc [45] | between 126 and 162 c |
| Tinta Miuda [57] | 2.2 a |
| Touriga Francesa [56] | 52.8 a |
| Viosinho [56] | 37.8 a |
| Methyl Cellulose Precipitation | |
| Tempranillo [42] | 24.29 |
a Unit mg/g FM. b mg CyE/100g DM. c mg CAE/g DM. d According to the extraction method.
Based on the values obtained by the Bate–Smith method (expressed in mg/g DM), proanthocyanidins appear to be the most abundant type of polyphenols in stem extracts. The dimer concentrations shown in Table 7 (expressed in mg/kg DM) represent only a small proportion of the proanthocyanidin content. The high values of total proanthocyanidin content suggest an abundance of polymerised forms, which is confirmed by the results of the mean degree of polymerization (mDP), which was found to be higher than 4.6 for all studied grape varieties (Table 9).
Table 9.
Mean degree of polymerization (mDP) and structural composition of stem polymeric proanthocyanidins.
| Grape Variety | mDP | General Composition | Terminal Units | Extension Units | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Cat | % Ec | % EcG | % EgC | % Cat | % Ec | % EcG | % EgC | % Cat | % Ec | % EcG | % EgC | |||
| Cabernet sauvignon [31] | Phloroglucinolysis Method | 5.9 | 25 | 74 | 1.0 | 97 | tr | 3 | 11 | 89 | ||||
| Callet [31] | 4.7 | 29 | 70 | 1.0 | 89 | 7 | 4 | 12 | 88 | |||||
| Chardonnay [31] | 4.6 | 28 | 71 | 1.0 | 89 | 6 | 5 | 11 | 89 | |||||
| Macabeu [31] | 6.2 | 24 | 75 | 1.0 | 83 | 11 | 6 | 13 | 87 | |||||
| Manto Negro [31] | 5.8 | 26 | 73 | 1.0 | 97 | tr | 3 | 11 | 89 | |||||
| Merlot [31] | 6.0 | 25 | 75 | 0.0 | 97 | tr | 3 | 10 | 90 | |||||
| Parellada [31] | 5.0 | 27 | 72 | 1.0 | 95 | 2 | 3 | 10 | 90 | |||||
| Premsal blanc [31] | 8.5 | 25 | 74 | 1.0 | 95 | tr | 4 | 16 | 84 | |||||
| Syrah [31] | 6.1 | 22 | 77 | 1.0 | 97 | tr | 3 | 7 | 93 | |||||
| Tempranillo [31] | 6.9 | 20 | 79 | 1.0 | 95 | tr | 5 | 8 | 92 | |||||
| Stems Vitis vinifera sp. [55] | Thiolysis Method | 5 | 16.8 | 55.3 | 17.1 | 10.5 | 84.2 | 11.3 | 4.5 | n.d. | 6.5 | 62.3 | 19.1 | 12.2 |
| Commercial stem powder [55] | 6.6 | 23.7 | 59.3 | 8.0 | 8.9 | 100.0 | n.d. | n.d. | n.d. | 13.5 | 67.3 | 9.1 | 10.1 | |
| Chardonnay [40] | 9.1 | 14 | 69.4 | 15.7 | 0.8 | |||||||||
| Clairette [40] | 7.7 | 17.3 | 68.4 | 13.4 | 0.9 | |||||||||
| Merlot [40] | 9.2 | 14.4 | 67.7 | 15.6 | 2.4 | 8.6 | 1.8 | 0.6 | 5.8 | 65.8 | 15.0 | 2.4 | ||
| Négrette [40] | 10.2 | 11.7 | 61.7 | 21.1 | 5.4 | |||||||||
| Pinot [40] | 8.2 | 15.3 | 65.1 | 18.1 | 1.5 | |||||||||
| Tannat [40] | 8.7 | 13 | 65.5 | 19.8 | 1.7 | |||||||||
mDP = Mean degree of polymerization; Cat = catechin; Ec = epicatechin; EcG = epicatechin gallate; Egc = epigallocatechin.
Thiolysis or phloroglucinolysis are used to analyze the condensed tannin composition. These reactions are depolymerization methods that cut the polymers into subunits. Only the extension unit forms adducts with the reactive, allowing for differentiating them from terminal units. The different monomers can be separately quantified by HPLC and the mean degree of polymerization can be determined. Results reported in the literature are presented in Table 9. The experimental values of the mean degree of polymerization (mDP) range from 4.6 to 10.2. The general composition shows that epicatechin is the main unit of the polymerized proanthocyanidins; it is also mainly found in extension units, whereas catechin is mostly found in terminal units. Merlot and Chardonnay were studied in two different papers, with different analysis methods, and the mDP were slightly different; higher for Souquet et al. than for Gonzalez-Centeno et al. [31,40]. This difference could be explained by vintage conditions, vine location, and analytical techniques. Sensitive difference can be observed between the two methods; EcG and EgC were found in higher concentrations using the thiolysis method than using phloroglucinolysis.
Anthocyanins
Anthocyanins are mainly located in grape skins [2,30]. However, recent studies analyzing different grape varieties identified some anthocyanin compounds in grape stem extracts: malvidin-3-O-glucoside [47,48], malvidin-3-O-(6-O-caffeoyl)-glucoside [47], malvidin-3-O-galactoside [48], and malvidin-3-O-rutinoside [47]. Total anthocyanin content of the stem extracts ranged from 0.06 to 1.4 mg/g of DM, and these compounds were not detected in some varieties. The concentration in anthocyanins was low compared to other flavonoid contents.
2.3.4. Impact of Polyphenolic Composition
Polyphenolic compounds have been widely studied and, apart from their influence on wine color and structure, they can influence different parameters, such as astringency or antioxidant activity.
Astringency
Astringency produces a contraction of the buccal mucosa when salivary proteins form complexes with tannins. Salivary amylase reacts strongly with astringent compounds and causes the mouth dryness sensation.
The influence of grape stem extracts’ polyphenolic composition on astringency has been studied using ovalbumin as a precipitation agent and tannic acid as a standard. Ovalbumin mimics the salivary proteins and quantifies astringency related to the precipitation of polyphenolic compounds and saliva. The results showed that stem extract astringency increases with maceration time and remains stable after 4–5 days [43]. Three maturation stages were studied, and ripening appeared to increase proanthocyanidin extraction during maceration and decrease the astringency of the extracts of all cluster parts. One hypothesis for the decreased astringency was a decrease in the mDP of proanthocyanidin extracted during stem ripening [43]. The relation between quantity, mDP, percent galloylation, and percent trihydroxylated units of proanthocyanidin and astringency has been studied in many wines and seed extracts [43,50,65,66]. In red wines, weaker astringency was found for lower mDP. In addition, the mDP is an average of the degree of polymerization and does not give clear indications of the proportion of polymeric and oligomeric proanthocyanidin content. Li et al. showed that polymeric polyphenols react more strongly with salivary proteins than oligomeric ones, inducing a higher sensation of astringency. During the ripening of the stems, the proportion of oligomeric forms may increase and could explain the decrease in astringency. The variation in proanthocyanidins, according to grape variety and stem ripening stage, appears to have a great influence on sensorial perception, especially regarding astringency. It would be interesting to study this parameter further when stems are kept during winemaking.
Antioxidant Activity
The antioxidant potential of polyphenolic compounds can be measured by different methods [67]. Studies have used different measurement techniques to characterize the antioxidant potential of stem extracts, such as 2,2-azinobis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), 1,1-diphenyl-2-picrylhydrazine (DPPH), ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC) [31], oxygen radical absorbance capacity (ORAC) [31], and superoxide radical scavenging activity (O2·-) [47]. ABTS, FRAP, and DPPH appeared to be the most used methods. Antioxidant capacity measured by DPPH can be expressed in different ways: either as the quantity of antioxidant necessary to decrease the concentration of initial DPPH or using a reference such as trolox. Summarizing the available data and comparing the values is not straightforward. Therefore, only the ABTS and FRAP values found in the literature are presented in Table 10.
Table 10.
Antioxidant activity of grape stem extracts (ABTS and FRAP methods).
| Grape Variety | ABTS | FRAP |
|---|---|---|
| Unit: mg Trolox/g DM | ||
| Arinto [48] | 87.6 | 87.6 |
| Cabernet Sauvignon [31] | 168.9 | 114.8 |
| Callet [31] | 253.2 | 170.1 |
| Castelao [48] | 115.1 | 140.2 |
| Chardonnay [31] | 99.7 | 65.4 |
| Fernao Pires [44] | 150.2 | |
| Fernao Pires [48] | 172.7 | 247.8 |
| Macabeu [31] | 131.7 | 85.5 |
| Malvasia fina [44] | 275.3 | |
| Manto Negro [31] | 198.2 | 134.6 |
| Merlot [31] | 109.8 | 76.6 |
| Moscatel [44] | 300.3 | |
| Parellada [31] | 223.4 | 159.1 |
| Premsal blanc [31] | 218.5 | 169.1 |
| Rabigato [44] | 250.3 | |
| Syrah [31] | 203.1 | 155.3 |
| Syrah [48] | 147.7 | 212.7 |
| Tempranillo [31] | 186.8 | 127.4 |
| Tinta Roriz [48] | 175.2 | 235.3 |
| Touriga Nacional [48] | 210.2 | 257.8 |
| Viosinho [44] | 200.2 | |
| Unit: mM Trolox/100 g DM | ||
| Amarela [47] | 57 | 37 |
| Fernao Pires [47] | 31 | 25 |
| Mazuelo [38] | 8 to 30 a | 4 to16 a |
| Moscatel (Sanfins du Douro) 2017 [33] | 38 | 33 |
| Moscatel (Sanfins du Douro) 2018 [33] | 67 | 74 |
| Moscatel (Penajóia) 2017 [33] | 73 | 84 |
| Moscatel (Penajóia) 2018 [33] | 73 | 85 |
| Moscatel (Medrões) 2017 [33] | 41 | 41 |
| Moscatel (Medrões) 2018 [33] | 69 | 75 |
| Rabigato [47] | 32 | 20 |
| Sousao [47] | 70 | 46 |
| Tinta Barroca [47] | 59 | 40 |
| Touriga Nacional [47] | 50 | 30 |
| Viosinho [47] | 40 | 24 |
a According to the extraction method.
As mentioned by Gonzalez-Centeno et al. it is difficult to compare the values reported in the literature because there is no standardized method to characterize the antioxidant potential; extracts are obtained using different techniques and the results are expressed in different units [31]. According to the literature, ABTS and FRAP results usually show good positive correlation [31,68,69,70,71,72]. Despite the difficulties of cross-study comparisons, all studies reported that stems can be a good source of antioxidant compounds.
2.4. Aromatic Composition
The use of stems during winemaking has been reported to bring vegetal and green aromas to the wine. Different studies focused on the aromatic compound found in stem extracts. In 1997, Hashizume et al. listed eight different green odorant compounds detected in grape stems from Cabernet Sauvignon and Chardonnay grape varieties: hexanal, (E)-2- hexanal, (Z)-1,5-octandien-3-one, 2-methoxy-3-isopropylpyrazine, 2-methoxy-3-isobutylpyrazine, dodecanal, (E,Z)-2,6-nonadienal, and an unknown compound (Table 11) [73]. These compounds were the same in both grape varieties. For Cabernet Sauvignon, four other aromas (a cooked vegetable-like odorant, a burned bamboo-like odorant, a sweaty unpleasant odorant, and a floral aroma) were also found during the extract analysis but were not analyzed because the study focused on vegetal aromas. Quantifying each compound showed that (Z)-1,5-octandien-3-one was the main green odorant compound from stems. These extracts were also compared to leaf, berry, and skin + seed extracts and stems appeared to contain the highest proportion of methoxypyrazine. Roujou de Boubée et al. focused on 2-methoxy-3-isobutylpyrazine (IBMP) in the Cabernet Sauvignon grape variety to determine the localization of this aromatic compound within the grape cluster, and found that stems were richer in IBMP, confirming the results of the Hashizume research team [73,74]. They also studied IBMP location during ripening and showed that it decreases in stems and seeds but increases in skins. Matarese et al. studied the entire fraction of volatile compounds of ground stems and other grape plant parts and reported that geraniol and geranic acid were the two main monoterpenes of the stem volatile fractions [75]. Other compounds, such as linalool and nerol, were also identified but in smaller proportions.
Table 11.
Identification of aromatic compounds in grape stem extracts.
| Grape Variety | Compound | Retention Index | Odor Description | ||
|---|---|---|---|---|---|
| DB-WAX | Ultra-1 | DB-5 | |||
| Cabernet Sauvignon and Chardonnay [73] | hexanal | 1099 | 800 | green | |
| (E)-2- hexanal | 1200 | 844 | green | ||
| (Z)-1,5-octandien-3-one | 1346 | 963 | geranium-like, metallic green | ||
| 2-methoxy-3-isopropylpyrazine | 1394 | 1092 | grassy, earthy | ||
| unknown | 1484 | - | cucumber-like | ||
| 2-methoxy-3-isobutylpyrazine | 1500 | 1211 | herbaceous, earthy | ||
| (E,Z)-2,6-nonadienal | 1561 | 1150 | cucumber-like | ||
| dodecanal | 1737 | 1402 | citrus skin -like | ||
| Syrah [76] | 3-isobutyl-2-methoxypyrazine | 1530 | 1184 | green pepper | |
| γ-octalactone | 1877 | 1276 | sweet, almond, coconut | ||
| trans-4,5-epoxy-E-2-decenal | 2011 | 1381 | metallic | ||
| furaneol (2.5-dimethyl-4-3(2H)-furanone) | 2036 | 1073 | caramel, red fruit jam aroma | ||
| eugenol (4-allyl-2-methoxyphenol) | 2171 | 1365 | clove | ||
| sotolon (3-hydroxy-4.5-dimethylfuran-2(5H)-one | 2198 | 1105 | curry | ||
| vanillin | 1564 | 1407 | vanilla | ||
Ruiz-Moreno et al. performed a GC-olfactometry and a GC-MS on Syrah stem extracts and found more than 80 odorant zones (OZ) [76]. Among them, eight OZ were found to be predominant and GC-MS identified the responsible molecules (Table 11). This study specified that stem extracts have a similar composition to that of wine in terms of aromatic compounds and should have a quantitative rather than qualitative effect if added to the wine.
In 2016, a study of Cabernet Sauvignon stems found a large amount of 1,8-cineole in the stems compared to the grape berries, and that this quantity substantially decreased during ripening [77]. Larger amounts of 2-methoxy-3-isopropylpyrazine (IPMP) and IBMP were found in the stems than in the grape berries. Again, these amounts decreased with ripening. Finally, this study identified methyl salicylate, which is reported to have a fresh and minty aroma and has higher levels in stems than berries (250 times higher).
Stems appear to be a rich source of valuable compounds, including polyphenols. Considering waste in wine production, their availability is high at harvest time and, as reported in the literature, grape stems may provide a cheap source of these compounds of interest. Recent studies have examined stem extracts for human health applications [44,48,78]. Although the destemming technique is widespread, some winemakers keep the stems during the winemaking process.
3. The Use of Stems during Winemaking
As explained in the introduction, in most cases of white winemaking, stems are kept for pressing and removed with the pomace. Because stems act as drains, keeping them during pressing induces better juice extraction. According to the literature, it also limits the presence of the thermo-unstable proteins responsible for protein breakdown [1]. For red winemaking, the impact of keeping the stems during fermentation and maceration appears to be more empirical. Understanding which elements are transferred and what impact they have on the wine is essential for advising winegrowers on this practice.
When stems are included, the whole cluster addition is the most common technique used. However, to precisely study the impact of stems, researchers have often added the stems back in the tank after destemming [3,12,30,43,57,73,79,80,81,82,83,84]. Different proportions of stems and whole clusters [81,83,84,85], different maceration time [30,43,79], and different stem pretreatments [3] have been used. Some studies also tested the use of stem powder [39] or stem extracts [76,86] as an oenological additive compound.
3.1. Impact on the Winemaking Process
Adding stems or using whole clusters can increase the must volume by 30%, which has a technological impact on the vatting and the maceration phases. In addition, a higher pressuring capacity is required [1].
To our knowledge, the impact of stems on alcoholic and malolactic fermentations (MLF) is barely described in the literature. Comparing destemmed and full-clustered musts has shown that must containing stems start fermenting faster, resulting in a wine with fewer residual sugars [1]. This may result from the structural configuration of stems, which allows higher incorporation of oxygen in the must, encouraging yeast proliferation. Moreover, the presence of such structures acting as temperature buffers could reduce temperature variations and hence prevent stuck fermentations. These effects are different depending on the volume of whole clusters or stems added. A recent study showed that 20% of whole clusters or 3% of stem weight in the vat did not influence either the temperature or the alcoholic fermentation kinetics [84]. For both fermentations, further studies are needed to better understand the impact of stems on microbial activity and kinetics.
3.2. Impact on the Main Wine Compounds
Stems release compounds, such as must [43,57,80,81], in a matrix, which then interact with the other grape-extracted compounds (from berries and seeds) and provoke a change in the overall balance of the final wine. Among the different types of compounds found in wines, some are widely influenced by the presence of stems, including ashes, acids, alcohols, and phenolic compounds.
3.2.1. pH and Acid Composition
Summarized data on the pH and acidic composition of wine made with and without stem addition are presented in Table 12.
Table 12.
pH and acidic composition of wine made with and without stem addition (for each study, different letters indicate significant statistical differences).
| Grape Variety | Modality | Maceration Time (Days) | pH | Titrable Acidity (g/L) | Total Acidity (g/L Tartaric Acid) | Volatile Acidity (g/L Acetic Acid) | Tartaric Acid (g/L) | Malic Acid (g/L) | Lactic Acid (g/L) | Acetic Acid (mg/L) | Phosphoric Acid (mg/L) | Citric Acid (mg/L) | Succinic Acid (g/L) | Shikimic Acid (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cabernet sauvignon [3] | no stem | 7 | 3.78 | 1.446b | 4.220 | 0.322 | 161 | 795a | 571a | 1.576 | ||||
| stem addition | 3.85 | 1.308a | 4.303 | 0.304 | 165 | 948b | 633b | 1.486 | ||||||
| Cabernet sauvignon [12] | no stem | 15 | 3.23a | 5.5b | ||||||||||
| stem addition | 3.45b | 4.7a | ||||||||||||
| Castelao [79] | no stem | 7 | 3.19 | 7.25 | 0.48 | 2.7b | 1.700 | 0.0 | ||||||
| stem addition | 3.22 | 7.15 | 0.47 | 2.6a | 1.700 | 0.0 | ||||||||
| no stem | 21 | 3.19a | 7.25 | 0.48 | 2.7b | 1.700 | 0.0a | |||||||
| stem addition | 3.28b | 6.55 | 0.49 | 2.5a | 0.900 | 0.300b | ||||||||
| Merlot [3] | no stem | 7 | 3.54 | |||||||||||
| stem addition | 3.54 | |||||||||||||
| Muscat bailey A [3] | no stem | 7 | 3.83a | 1.105b | 3.787 | 0.343 | 276 | 669a | 729 | 1.228b | ||||
| stem addition | 3.95b | 1.011a | 3.790 | 0.382 | 247 | 926b | 767 | 1.032a | ||||||
| Pinot Noir [3] | no stem addition | 7 | 3.60a | |||||||||||
| stem addition | 3.69b | |||||||||||||
| Pinot Noir 2014 [84] | no stem | 10 | 3.74 | |||||||||||
| stem addition | 3.74 | |||||||||||||
| Pinot Noir 2015 [84] | no stem | 10 | 3.66bc | 4.03b | 0.90b | 0.760 | 1.31 | |||||||
| stem addition | 3.71c | 3.86a | 0.94b | 0.660 | 1.30 | |||||||||
| 20% whole cluster | 3.63ab | 3.96a | 0.85a | 0.810 | 1.31 | |||||||||
| Pinot Noir 2016 [83] | no stem addition | 10 | 3.36b | 6.4b | 0.77b | 0.05 | 0.86ab | |||||||
| 50% whole cluster | 3.55a | 6.8a | 0.81b | 0.05 | 0.81ab | |||||||||
| 100% whole cluster | 3.53a | 6.8a | 1.11a | 0.06 | 0.79b | |||||||||
| dried stems | 3.52a | 6.5ab | 0.85b | 0.06 | 0.87a | |||||||||
| Pinot Noir 2017 [83] | no stem addition | 10 | 3.31c | 7.1 | 0.79b | 0.04 | 0.58b | |||||||
| 50% whole cluster | 3.42bc | 7.2 | 0.95ab | 0.04 | 0.66ab | |||||||||
| 100% whole cluster | 3.60a | 7.2 | 1.12a | 0.04 | 0.76a | |||||||||
| dried stems | 3.51ab | 7.1 | 0.91ab | 0.06 | 0.72a | |||||||||
| Primitivo [81] | no stem | 10 | 3.91 | 6.15 | 0.75 | 1.60 | 1.73 | 0.04 | 1.14 | 1.28 | 18.4a | |||
| 25% whole cluster | 3.84 | 6.38 | 0.75 | 1.53 | 2.4 | 0.05 | 0.96 | 1.23 | 23.7b | |||||
| 50% whole cluster | 3.90 | 6.3 | 0.66a | 1.69 | 1.7 | 0.06 | 0.97 | 1.33 | 22.4b | |||||
| Tinta Miuda [80] | no stem | 6 | 3.0 | 8.6 | 0.8 | |||||||||
| stem addition | 3.0 | 8.5 | 0.7 |
Hashizume et al. found an increase in pH for Pinot Noir and Muscat Bailey A musts when incorporating the stems back in the vats [3]. This phenomenon was also observed by Pascual et al. on Cabernet Sauvignon musts and more recently by Casassa et al. during Pinot Noir winemaking using either whole clusters, raw or dried stems [12,83,84].
However, pH increases are not always significant. These differences in terms of variations may be linked to the high buffering capacity of wine matrices over acido-basic balance that mainly depends on the grape variety [50]. The impact of stem contact duration has also been studied. For Castelao musts, Spranger et al. reported that pH did not show a significant increase after seven days, but showed a significant impact after 21 days of contact [79]. Therefore, contact duration seems to influence pH variation.
According to the acid composition, titrable acidity was shown to be significantly lower than the control samples for Cabernet Sauvignon [12] and Pinot Noir wines going through stem contact [84]. However, this finding seems to depend on how the stems are incorporated in the Casassa et al. latest study; the use of whole clusters did not show a significant decrease in titrable acidity [83].
Changes in acid composition appear to be responsible for these pH variations in wine. More specifically, tartaric acid, which is the most abundant acid found in wines and musts, seems to be affected by the addition of stems, but less by the use of whole clusters (Table 12). After seven days of contact, its concentration was lowered by 4% for Castelao wines [79], 9% for Muscat Bailey A [3] and 10% for Pinot Noir wines [3]. With longer stem contact duration, the decrease in tartaric acid was greater (from 4% after seven days to 7% after 21 days for Castelao wines [79]). This loss could result from precipitation mechanisms of tartaric acid. As noted earlier, stems are rich in mineral compounds, especially potassium, so their interaction with tartaric acid is a possible explanation [12].
Other acid concentrations might also be affected by stem contact as a result of molecular interactions. For instance, lower concentrations of succinic acid were reported for Muscat Bailey A (−16%), whereas phosphoric acid concentration was increased by 38% [3]. Differences in lactic and malic acid concentrations mainly resulted from the winemaking process and whether the MLF was performed. Lastly, Casassa et al. highlighted the fact that the use of the whole cluster may increase the volatile acidity of the wine potentially due to undesirable bacterial growth taking place in the air spaces within the whole clusters [83].
3.2.2. Ashes
Only a few authors have studied the impact of stems on the mineral composition of wines. However, both Hashizume et al. and Sun et al. found significant increases in the concentration of several mineral ions [3,80]. The related values are summarized in Table 13.
Table 13.
Mineral composition of wines made with and without stem addition (mg/L) (for each study, different letters indicate significant statistical differences).
| Grape Variety | Modality | K | P | Ca | Mg | Na | Mn | Fe | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Cabernet Sauvignon [3] | no stem addition | 1454a | 198a | 62a | 71 | 4.4 | 0.6 | 5.8 | 0.5a | 0.3 |
| stem addition a | 1927b | 277b | 69b | 73 | 4.8 | 0.8 | 5.0 | 0.9b | 0.3 | |
| Muscat Bailey A [3] | no stem addition | 2046a | 208a | 74a | 55a | 4.7 | 0.8 | 5.1 | 0.9 | 0.5a |
| stem addition a | 2476b | 389b | 90b | 60b | 4.7 | 0.9 | 7.0 | 0.8 | 0.8b | |
| Tinta Miuda [80] | no stem addition | 1065.8a | 79.2a | 88.0a | 12.4a | 2.4 | 0.1 | |||
| stem addition b | 1088.6b | 104.0b | 96.0b | 20.0b | 2.0 | 0.1 |
Stems were left in contact with the must for: a 7 days; b 6 days.
Both authors found that stem addition increases potassium (K), phosphorus (P), and calcium (Ca) concentrations. This reinforces the hypothesis of tartaric acid precipitation inducing a pH increase.
Some variations were recorded depending on the grape varieties, especially for magnesium (Mg), sodium (Na), copper (Cu), and zinc (Zn), indicating that the impact of stems could also depend on the grape variety.
3.2.3. Ethanol Content
Several authors related the addition of stems to a lower wine ethanol content [12,80,84] (Table 14). Hashizume et al. attributed this decrease to a dilution phenomenon [3]. Indeed, stems have a high water content (see Section 2.2.1. Water), which could be transferred to the wine during maceration. Pascual et al. presented similar conclusions and added that the stem surface could also capture ethanol molecules by adsorption [12].
Table 14.
Ethanol content in wine made with and without stem addition (for each study, different letters indicate significant statistical differences).
| Grape Variety | Modality | Maceration time (Days) | Alcohol (% v/v) |
|---|---|---|---|
| Cabernet sauvignon [12] | no stem addition | 15 | ≈13b |
| stem addition | ≈12.6a | ||
| Castelao [79] | no stem addition | 7 | 13.3 |
| stem addition | 13.3 | ||
| no stem addition | 21 | 13.3 | |
| stem addition | 13.2 | ||
| Pinot Noir 2014 [84] | no stem addition | 7 | 13.03b |
| stem addition | 12.75a | ||
| Pinot Noir 2015 [84] | no stem addition | 10 | 15.16b |
| stem addition | 15.03b | ||
| 20% whole cluster | 14.31a | ||
| Pinot Noir 2016 [83] | no stem addition | 10 | 13.07 |
| 50% whole cluster | 13.24 | ||
| 100% whole cluster | 13.02 | ||
| dried stems | 13.48 | ||
| Pinot Noir 2017 [83] | no stem addition | 10 | 14.54ab |
| 50% whole cluster | 13.90b | ||
| 100% whole cluster | 14.24ab | ||
| dried stems | 14.68a | ||
| Primitivo [81] | no stem addition | 10 | 19.67b |
| 25% whole cluster | 19.38c | ||
| 50% whole cluster | 20.05a | ||
| Tinta Miuda [80] | no stem addition | 6 | 8.4b |
| stem addition | 7.7a |
Nevertheless, lower ethanol values are not always clearly observed. While comparing the impact of different technologies on the wine profile, Spranger et al. did not observe any real change when adding stems to the fermenting wines [79]. The impact of stems on alcohol content is not yet well understood; it might be interesting to compare these results with the moisture content of stems.
3.3. Impact on Polyphenolic Composition
3.3.1. Total Phenolic Compounds
Two methods were used to analyze the total phenolic fraction of wines: Folin Index (FI) and Total Polyphenol Index (TPI). The FI is based on the Folin–Ciocalteu method. TPI uses the typical properties of the benzenic structures found in phenolic compounds, which can absorb at 280 nm when measured by spectrometry. Even though this measure is not very accurate for quantification, it gives a good indication of the phenolic content in wines. Several types of phenolic compounds contribute to this index, such as anthocyanins and tannins, as well as a small fraction of non-phenolic compounds [50]. Data available in the literature are summarized in Table 15. For most of the grape varieties, the total phenolic content increased when stems were included during winemaking. Castelao is the only grape variety for which no significant difference was found. The magnitude of the variation seems to be correlated both to varietal differences and maceration duration.
Table 15.
Total polyphenol compounds in wine made with and without stem addition (for each study, different letters indicate significant statistical differences).
| Grape Variety | Modality | Maceration Time (days) | Total Phenolic Compounds (FI) (mg GAE/L) |
Total Polyphenol Index (TPI) |
|---|---|---|---|---|
| Cabernet sauvignon [12] | no stem addition | 15 | 42.0a | |
| stem addition | 48.2b | |||
| Cabernet sauvignon [3] | no stem addition | 7 | 1769a | |
| stem addition | 2160b | |||
| Castelao [79] | no stem addition | 7 | 46.2 | |
| stem addition | 50.0 | |||
| no stem addition | 7 | 46.2 | ||
| stem addition | 21 | 49.0 | ||
| Merlot [3] | no stem addition | 7 | 1483a | |
| stem addition | 1923b | |||
| Muscat bailey A [3] | no stem addition | 7 | 1334a | |
| stem addition | 1671b | |||
| Pinot Noir [3] | no stem addition | 7 | 1013 | |
| stem addition | 1100 | |||
| Primitivo [81] | no stem addition | 10 | 2685a | |
| 25% whole cluster | 3127b | |||
| 50% whole cluster | 3164b | |||
| Tinta Miuda [80] | no stem addition | 6 | 26.47a | |
| stem addition | 32.19b |
3.3.2. Non-Flavonoid Compounds
Even if some interesting non-flavonoid compounds were found in the grape stem extracts, their transfer and presence in wines has not been thoroughly investigated. Pascual et al. examined hydroxycinammic acid derivatives in Cabernet sauvignon wines, and Benitez et al. in Palomino fino [12,85]. These compounds were measured using reversed-phase HPLC, diode array detector, electrospray ionization, and tandem mass spectrometry systems (HPLC-DAD-ESI-MS). Overall, the tested wines were not significantly affected by stem contact in terms of phenolic acid content. Caftaric and gallic acids that were the main phenolic acids found in grape stem extracts does not seem to be significantly transferred to wine (Table 16).
Table 16.
Impact of stems on phenolic acids composition of the wine (mg/L) (for each study, different letters indicate significant statistical differences).
| Grape Variety | Modality | Maceration Time (Days) | Gallic Acid | Syringic Acid | Caftaric Acid | 2-S-Gluthationyl Caftaric Acid | trans p-Coutaric Acid | cis p-Couratic Acid | Fertaric Acid | Caffeic Acid | Trans p-Coumaric Acid | Ferulic Acid |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palomino fino [85] | 100% whole cluster | 9 | 2.40 | 1.44a | 36.98 | 7.38 | 9.08 | 3.49 | 0.65 | 5.09a | 0.38 | 0.32 |
| 75% whole cluster | 9 | 10.47 | 1.76 | 40.95a | 10.12 | 9.49b | 3.62 | 0.65 | 2.82b | 0.41b | 0.62 | |
| 50% whole cluster | 9 | 6.08 | 1.20 | 37.17b | 8.36 | 9.24a | 3.49a | 0.64 | 4.27 | 0.27b | 0.35 | |
| 25% whole cluster | 9 | 3.29 | 1.82b | 38.57 | 8.53 | 10.1 | 4.27b | 0.86 | 5.00 | 0.56a | 0.41 | |
| Cabernet sauvignon [12] | no stem addition | 15 | 18.38 | 1.03 | 0.62 | 0.45 | 2.12 | |||||
| stem addition | 20.24 | 1.03 | 0.72 | 0.50 | 2.20 |
Although the concentration of stilbene and stilbenoid compounds has been extensively studied for the antioxidant properties in the stem extracts, to our knowledge, their transfer from the stem to the wine has not been studied. However, in their study, Bavaresco et al. mimicked alcoholic fermentation, using an hydroalcoholic solution (11% (v/v) ethanol and 250 ppm (v/v) methanol) as an extraction solvent, in order to quantify the content of potentially extractable stilbenes [51]. Their results showed that only trans-resveratrol was extracted. For this experiment, the ethanol content remained constant during the extraction. It would be interesting to carry out the same study with an increasing concentration of ethanol and also in fermenting must in order to valid the transfer of these compounds to the wine.
3.3.3. Flavonoid Compounds
To our knowledge, the impact of stem contact on flavones or flavanones content has not been reported in the literature. Pascual et al. [12] studied the impact of stem contact on the flavonol content of Cabernet Sauvignon wine. Their results showed a significant decrease in total flavonols, mainly resulting from aglycones, and they suggested that stems might absorb these compounds. Further study is needed to confirm this hypothesis. Apart from this study, no further information was found in the literature.
Flavan-3-ols and Proanthocyanidins
Total proanthocyanidin content results are shown in Table 17 and individual flavan-3-ol and proanthocyanidin composition results are shown in Table 18. The total proanthocyanidin content seems to significantly increase when either stems or whole clusters are kept during maceration, regardless of the grape variety. According to Casassa et al.’s latest study on Pinot Noir wines, the increase seems to be more or less correlated to the amount of stems in the vat, whether fresh or dry [83]. This observation is also valid for Suriano et al.’s results on Primitivo wines [81].
Table 17.
Impact of stems during winemaking on total proanthocyanidin content (for each study, different letters indicate significant statistical differences).
| Grape Variety | Vintage | Modality | Maceration Time (days) | Total Proanthocyanidin (mg/L) |
|---|---|---|---|---|
| Method: Bate-Smith Reaction | ||||
| Cabernet [30] | 1966 | no stem addition | 4 | 1700 |
| stem addition | 2100 | |||
| Cabernet [30] | 1966 | no stem addition | 20 | 4200 |
| stem addition | 4500 | |||
| Cabernet [30] | 1967 | no stem addition | n.d. | 1700 |
| stem addition | 2000 | |||
| Malbec [30] | 1966 | no stem addition | 4 | 2400 |
| stem addition | 3500 | |||
| no stem addition | 8 | 3200 | ||
| stem addition | 3900 | |||
| no stem addition | 14 | 3500 | ||
| stem addition | 4500 | |||
| no stem addition | 30 | 3700 | ||
| stem addition | 4700 | |||
| Merlot [30] | 1966 | no stem addition | 8 | 2500 |
| stem addition | 4000 | |||
| Method: Vanillin Assay | ||||
| Primitivo [81] | 2012 | no stem addition | 10 | 1744a |
| 25% whole cluster | 2180b | |||
| 50% whole cluster | 2275b | |||
| Method: Precipitation Methods | ||||
| Cabernet sauvignon 1 [12] | 2013 | no stem addition | 15 | 403a |
| stem addition | 778b | |||
| Pinot Noir 2 [84] | 2014 | no stem addition | 10 | 370 |
| 20% whole cluster | 350 | |||
| Pinot Noir 2 [84] | 2015 | no stem addition | 10 | 540b |
| stem addition | 860c | |||
| 20% whole cluster | 440a | |||
| Pinot Noir 2 [83] | 2016 | no stem addition | 10 | 100a |
| 50% whole cluster | 210b | |||
| 100% whole cluster | 320c | |||
| dried stems | 325c | |||
| Pinot Noir 2 [83] | 2017 | no stem addition | 10 | 112a |
| 50% whole cluster | 175a | |||
| 100% whole cluster | 270c | |||
| dried stems | 275c | |||
1 Methyl cellulose precipitation. 2 Protein precipitation (mg/L catechin equivalent (CE).
Table 18.
Impact of stems during winemaking on flavan-3-ol monomeric and polymeric composition.
| Grape Variety | Vintage | Modality | Maceration Time (Days) | Cat | Ec | Gc | EcG | Egc | EgcG | B1 | B2 | B3 | B4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cabernet sauvignon [12] | 2013 | stem addition | 15 | ++ | 0 | ++ | ++ | ||||||
| Castelao [79] | 2000 | stem addition | 7 | + | 0 | + | 0 | 0 | 0 | ||||
| stem addition | 21 | ++ | ++ | 0 | 0 | 0 | 0 | ||||||
| Primitivo [81] | 2012 | 25% whole cluster | 10 | + | + | − | 0 | + | + | + | + | + | ++ |
| 50% whole cluster | 10 | ++ | ++ | −− | + | + | + | ++ | + | +++ | ++ | ||
| Tinta Miuda [57] | 1996 | stem addition | 21 | ++ | ++ | 0 | 0 | 0 | 0 | ||||
| Tinta Miuda [80] | 1998 | stem addition | 6 | +++ | − | +++ | 0 | +++ | 0 |
Cat = catechin; Ec = epicatechin; Gc = gallocatechin; EcG = epicatechin gallate; Egc = Epigallocatechin; EgcG = Epigallocatechine gallate; B1, 2, 3 and 4 = proanthocyanidins dimers; % of variation: 0–50 (+/−); 50–100 (++/−−); 100–250 (+++/−−−); 250–500 (++++/−−−−); >500(+++++/−−−−−).
According to the flavan-3-ol monomeric and polymeric composition, the intensity of the variations differed across studies. It is hard to draw conclusions from these values because stem proportions added for alcoholic maceration and maceration duration varied. However, there was a tendency for higher concentrations of catechins, epicatechin, dimer B1 and B3 in nearly every study. For dimers B2 and B4, the concentration variation did not seem to depend on either the grape variety or the maceration duration.
Suriano et al. reported a clear increase in the concentration of catechin, epicatechin, epicatechin gallate, and procyanidins B1 and B3 that evolved to the quantity of full clusters present in the wines [81]. Conversely, gallocatechin and procyanidin T2 (not shown here) concentrations decreased when stems were added, which could be linked to adsorption by stem bodies or interactions with wine molecules.
Little information is available regarding the impact of stems on proanthocyanidin mDP [12,79,80]. Available results did not show significant difference induced by stem contact (data not shown).
Anthocyanins
According to several authors, stem contact during maceration decreased total anthocyanin concentration [12,30,79,80,81] (Table 19). Spectrophotometric measurements to determine anthocyanin concentration are possible thanks to chemical methods based on anthocyanin color properties. pH variation methods, such as the Puissant–Leon method are based on matrix acidification by HCl that cause a change in anthocyanin color [50,87]; the SO2-bleaching method is based on the discoloration of anthocyanins in the presence of sulfur dioxide [88]. These measurement techniques are only partially accurate, because they only quantify the sum of free anthocyanin and the part of the combined anthocyanins fraction that is sensitive to sulfur-dioxide bleaching [50]. However, among the wines tested with the same method, anthocyanin concentration decreases seemed proportional with the stem contact maceration duration, with Malbec wines studied by Ribéreau-Gayon and Mihlé the only exception; anthocyanin content decreased with stem addition but not proportionally to the contact duration [30]. When whole grape clusters were used, the concentration in anthocyanin was even lower and tended to decrease with an increasing proportion of full clusters [79,80,81]. Although molecular interactions between compounds from the stems and the musts could explain part of this anthocyanin loss, Ribéreau-Gayon and Mihlé rejected this hypothesis, because the addition of stem extract did not affect the anthocyanin concentration [30]. Instead, the authors explained this loss by the adsorption phenomenon provoked by the stem bodies on the anthocyanin molecules, similar to the explanation given by other authors [81]. However, this finding was not found in Casassa et al.’s latest study [83]. The anthocyanin content of wine made of 50% and 100% of whole cluster was not significantly different from the control wine (fully destemmed). The authors highlighted that the vintage conditions can have more of an impact on the anthocyanin content than the winemaking process; an additional argument shows that it would be relevant to evaluate and take into consideration the maturity of the stems.
Table 19.
Impact of stems during winemaking on total anthocyanin content (for each study, different letters indicate significant statistical differences).
| Grape Variety | Vintage | Modality | Maceration Time (Days) | Total Anthocyanin (mg/L) |
|---|---|---|---|---|
| Method: pH Variation—HCl | ||||
| Primitivo [81] | 2012 | no stem addition | 10 | 401 1 a |
| 25% whole cluster | 374 1 b | |||
| 50% whole cluster | 368 1 b | |||
| Cabernet sauvignon [12] | 2013 | no stem addition | 15 | 474.5 3 b |
| stem addition | 426.4 3 a | |||
| Method: SO2 Bleaching | ||||
| Cabernet [30] | 1966 | no stem addition | 4 | 800 2 |
| stem addition | 690 2 | |||
| Cabernet [30] | 1966 | no stem addition | 20 | 800 2 |
| stem addition | 690 2 | |||
| Cabernet [30] | 1967 | no stem addition | n.d. | 710 2 |
| stem addition | 700 2 | |||
| Castelao [79] | 2000 | no stem addition | 7 | 283 3 |
| stem addition | 261 3 | |||
| no stem addition | 21 | 283 3 b | ||
| stem addition | 221 3 a | |||
| Malbec [30] | 1966 | no stem addition | 4 | 630 2 |
| stem addition | 570 2 | |||
| no stem addition | 8 | 610 2 | ||
| stem addition | 500 2 | |||
| no stem addition | 14 | 600 2 | ||
| stem addition | 540 2 | |||
| no stem addition | 30 | 390 2 | ||
| stem addition | 320 2 | |||
| Merlot [30] | 1966 | no stem addition | 8 | 5402 |
| stem addition | 580 2 | |||
| Pinot Noir [84] | 2014 | no stem addition | 10 | 270 3 |
| 20% whole cluster | 260 3 | |||
| Pinot Noir [84] | 2015 | no stem addition | 10 | 250 3 |
| stem addition | 270 3 | |||
| no stem addition | 10 | 250 3 | ||
| 20% whole cluster | 280 3 | |||
| Pinot Noir [83] | 2016 | no stem addition | 10 | 251 3 |
| 50% whole cluster | 250 3 | |||
| 100% whole cluster | 251 3 | |||
| dried stems | 251 3 | |||
| Pinot Noir [83] | 2017 | no stem addition | 10 | 150 3 |
| 50% whole cluster | 140 3 | |||
| 100% whole cluster | 135 3 | |||
| dried stems | 150 3 | |||
| Tinta Miuda [80] | 1998 | no stem addition | 6 | 148.77 3 b |
| stem addition | 129.72 3 a | |||
1 mg/L malvidin chloride. 2 mg/L unspecified reference. 3 mg/L malvidin-3-O-glucoside.
Some authors studied the individual anthocyanin composition by HPLC analysis (Table 20). Anthocyanin 3-monoglucosides, which are the major anthocyanins in the tested wines, were the most affected by stem addition, where the malvidin-3-O-glucoside counted as more than 50% of the fraction. Studies led by Spranger et al. showed a 19.6% decrease in its concentration after seven days of stem contact, and this decrease was even more important (30.2%) with extended stem contact (21 days) [79]. Similar results were reported by Sun et al. and Suriano et al. who found decreases of about 17 to 18% [80,81]. A decrease in anthocyanin 3-monoglucosides was also reported by Pascual et al. but to a lesser extent (6.0%) [12]. Furthermore, the proportion of stems added had a smaller impact on the anthocyanin 3-monoglucoside concentration than the stem contact duration.
Table 20.
Impact of stems during winemaking on individual anthocyanin content (mg/L) (for each study, different letters indicate significant statistical differences).
| Grape Variety | Vintage | Modality | Maceration Time (Days) | Delphinidin-3-O-G | Cyanidin-3-O-G | Petunidin-3-O-G | Peonidin-3-O-G | Malvindin-3-O-G | Acetylated Anthocyanins | P-Coumarylated Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Standard: Malvidin Chloride | ||||||||||
| Primitivo [81] | 2012 | no stem addition | 10 | 5.67a | 0.77a | 15.31a | 8.72a | 181.37a | 19.6b | 26.44a |
| 25% whole cluster | 4.42b | 0.66b | 12.6b | 7.51b | 150.50b | 21.65a | 22.98b | |||
| 50% whole cluster | 4.3b | 0.54c | 12.25b | 7.31b | 149.21b | 18.27b | 22.36b | |||
| Reference Standard: Malvidin-3-O-Glucoside | ||||||||||
| Cabernet sauvignon [12] | 2013 | no stem addition | 15 | 27.2 | 11.6b | |||||
| stem addition | 27.0 | 8.9a | ||||||||
| Castelao [79] | 2000 | no stem addition | 7 | 10.8b | 1.6b | 13.5b | 19.1b | 115.9b | n.d. | 16.6b |
| stem addition | 9.1a | 1.4a | 10.9a | 15.6a | 91.9a | n.d. | 15.1a | |||
| no stem addition | 21 | 10.8b | 1.6b | 13.5b | 19.1b | 115.9b | n.d. | 16.6 | ||
| stem addition | 8.1a | 1.2a | 9.3a | 13.2a | 80.2a | n.d. | 12.3 | |||
| Pinot Noir [84] | 2014 | no stem addition | 10 | 4 | n.d. | 9 | 28 | 168 | ||
| 20% whole cluster | 4 | n.d. | 9 | 28 | 160 | |||||
| Pinot Noir [84] | 2015 | no stem addition | 10 | 4 | 3 | 8 | 24 | 96 | ||
| stem addition | 4 | 3 | 8 | 25 | 96 | |||||
| 20% whole cluster | 4 | 3 | 8 | 25 | 112 | |||||
| Tinta Miuda [80] | 1998 | no stem addition | 6 | 5.73b | 1.89b | 6.59b | 17.51b | 62.96b | 12.77 | 15.2 |
| stem addition | 5.19a | 1.86a | 5.93a | 13.94a | 51.52a | 11.81 | 13.15 | |||
Other types of anthocyanins, combined with specific molecules, were affected by stems, including p-coumarylated anthocyanins that showed decreasing patterns depending on stem contact duration [12,81]. However, the quantity of stems in contact with the must did not significantly lower their concentration. Decreases in acetylated anthocyanins did not always reach significance.
3.4. Impact on the Wine Sensorial Characteristics and Aging Potential
3.4.1. Color
Wine color measurements were mainly performed using color intensity calculation, summation of the absorbance at 420, 520, and 620 nm, and the hue ratio between absorbance at 420 and 520 nm. The results are shown in Table 21. Using stems during winemaking tended to decrease color intensity and increase hue, giving the wine a more reddish color, but this result was not always observed. In some cases, adding stems decreased the hue [30,81]. CIELAB measurements were also performed in some studies but results were not significantly different when stems were included during winemaking [79,80] (data not shown). Different explanations were offered for the color changes: dilution linked to stem water released in the must, pH modification allowing the transformation of anthocyanins into uncolored compounds, and possible adsorption of anthocyanin content by the stems [3,12,30]. The impact of stems on color intensity was more important for short maceration and tended to have no significant impact on long duration maceration [30].
Table 21.
Impact of stems on color intensity and hue of wine (for each study, different letters indicate significant statistical differences).
| Grape Variety | Vintage | Modality | Maceration Time (Days) | Color Intensity A420 + A520 + A620 | Hue A420/A520 |
|---|---|---|---|---|---|
| Cabernet [30] | 1966 | no stem addition | 4 | 1.51 | 0.42 |
| stem addition | 1.11 | 0.55 | |||
| Cabernet [30] | 1966 | no stem addition | 20 | 1.39 | 0.55 |
| stem addition | 1.24 | 0.43 | |||
| Cabernet [30] | 1967 | no stem addition | nd | 1.35 | 0.41 |
| stem addition | 1.21 | 0.51 | |||
| Cabernet sauvignon [3] | 1996 | no stem addition | 7 | 0.505a | |
| stem addition | 0.592b | ||||
| Cabernet sauvignon [12] | 2013 | no stem addition | 15 | 9.7b | 0.529a |
| stem addition | 8.1a | 0.583b | |||
| Malbec [30] | 1966 | no stem addition | 4 | 1.52 | 0.52 |
| stem addition | 1.22 | 0.56 | |||
| no stem addition | 8 | 1.62 | 0.56 | ||
| stem addition | 1.22 | 0.57 | |||
| no stem addition | 14 | 1.36 | 0.51 | ||
| stem addition | 1.33 | 0.59 | |||
| no stem addition | 30 | 1.2b | 0.67 | ||
| stem addition | 1.19 | 0.67 | |||
| Merlot [30] | 1966 | no stem addition | 8 | 1.41 | 0.55 |
| stem addition | 1.19 | 0.54 | |||
| Merlot [3] | 1996 | no stem addition | 7 | 0.630a | |
| stem addition | 0.717b | ||||
| Muscat Bailey A [3] | 1996 | no stem addition | 7 | 0.758a | |
| stem addition | 0.866b | ||||
| Pinot Noir [3] | 1996 | no stem addition | 7 | 1.020a | |
| stem addition | 1.133b | ||||
| Pinot Noir [84] | 2014 | no stem addition | 10 | 0.39 | |
| 20% whole cluster | 0.36 | ||||
| Pinot Noir [84] | 2015 | no stem addition | 10 | 0.6 | |
| stem addition | 0.64 | ||||
| no stem addition | 10 | 0.6 | |||
| 20% whole cluster | 0.56 | ||||
| Pinot Noir [83] | 2016 | no stem addition | 10 | 0.5 | |
| 50% whole cluster | 0.57 | ||||
| 100% whole cluster | 0.59 | ||||
| dried stems | 0.65 | ||||
| Pinot Noir [83] | 2017 | no stem addition | 10 | 0.58 | |
| 50% whole cluster | 0.52 | ||||
| 100% whole cluster | 0.57 | ||||
| dried stems | 0.6 | ||||
| Primitivo [81] | 2012 | no stem addition | 10 | 11.97a | 0.72b |
| 25% whole cluster | 15.9b | 0.67a | |||
| no stem addition | 10 | 11.97a | 0.72b | ||
| 50% whole cluster | 15.2b | 0.70a |
Other work reported increased color intensity despite lower anthocyanin content [81]; this was explained as stems bringing more oxygen to the must and promoting condensation between anthocyanins–tannins–acetaldehyde, which is important for color stability.
3.4.2. Aroma and Volatile Compounds
Spranger et al. and Benitez et al. studied the impact of various proportions of stems on the volatile fraction of wines [79,85]. With Castelao wines, 1-hexanol (grass odor) and ethyl decanoate (waxy odor) were the two compounds affected after 21 days of maceration with stems. Among all the other compounds identified in both studies, no significant effect of stem quantity was found on the concentration of molecules.
Among the 14 volatile compounds identified by Casassa et al. the concentration of β-damascenone, a nor-isoprenoid described as a fruity aroma enhancer, was higher when whole clusters where used during winemaking [84]. However, sensory analysis did not confirm these results because wines were described as less fruity. No green taste was identified in the wines, although compounds that give a green taste, such as 1-hexanol, isobutanol, or hexanoic acid, differed in concentration when stems were added. In Casassa et al. latest study, wines made of 100% whole clusters of grapes showed higher levels of ethyl cinnamate and benzaldehyde (spice and almond-like odor) and those in which dried stems were added exhibited higher levels of esters (potential fruity and floral odors) [83]. Sensory analysis confirmed these differences: 100% whole cluster wine had higher vegetal, cooked fruit flavors and spicy notes and wines made with dried stems had more herbal and fruity odors. Compounds known to bring a vegetal note to wine, such as methoxypyrazines, were not examined in this study.
IBMP is known to be easily extracted during pressing and maceration [74]. Because its concentration is high in stems, stem contact processes could increase IBMP levels in must and wine. Consistent with this hypothesis, Hashizume and Samuta identified methoxypyrazine compounds in wines from Cabernet Sauvignon and Chardonnay varieties fermented with and without stems [73]. Compared to wines made from fully destemmed bunches, stem contact wines had significantly higher concentrations of 2-methoxy-3-isopropylpyrazine (IPMP), 2-methoxy-3-sec-butylpyrazine (SBMP), and IBMP. Similar patterns were found in a more recent study on Sauvignon Blanc wines [82]. The results are presented in Table 22.
Table 22.
Impact of stems on methoxypyrazine composition in wine samples.
| Grape Variety | Modality | Methoxypyrazine Compounds | ||
|---|---|---|---|---|
| IPMP (ng/L) | SBMP (ng/L) | IBMP (ng/L) | ||
| Cabernet Sauvignon [73] | No stem addition | n.d. | n.d. | 25.3 |
| Stem addition | 2.7 | 2.8 | 33.8 | |
| Chardonnay [73] | No stem addition | n.d. | n.d. | 11.6 |
| Stem addition | 2.5 | 2.0 | 18.0 | |
| Sauvignon Blanc [82] | No stem addition | 0.85 | n.d. | 4.8 |
| Stem addition | 3.6 | 0.8 | 14.1 | |
n.d.: not detected; IPMP = 2-methoxy-3-isopropylpyrazine; SBMP = 2-methoxy-3-sec-butylpyrazine; IBMP = 2-methoxy-3-isobutylpyrazine.
More precisely, IBMP was the only compound detected without stem addition at concentrations greater than 1 ng/L, suggesting that IPMP and SBMP were introduced by the stem contact [73,82].
Although values were the same order of magnitude, differences in concentration reported in the literature could be linked to winemaking practices involving various stem quantities in contact with the must. Moreover, the number of wounds provoked during grape harvest, the lignification state of stems, and potential varietal effects could be responsible for these variations. Further work is needed to investigate the implication of each factor in methoxypyrazine concentration in stem-contact wines.
3.4.3. Taste
Studies that performed sensory analysis of wines made using either stems or whole clusters reported similar conclusions regarding bitterness and astringency. Pascual et al. reported that the stem significantly increased astringency and tended to increase the bitterness of Cabernet Sauvignon wines [12]. More recently, Casassa et al. reported similar results for Pinot Noir wines, for both fresh or dried stems [83,84]. In these two studies, the degree of stem lignification was not considered. It might be interesting to examine whether the sensory impact is different when the stems are lignified.
On the other hand, in the conclusions of their latest study, Casassa et al. wrote about the impact of stems on the “freshness” of the wines [83]. This effect is relatively well known from an empirical point of view. In this study, the wines were noted as fresher, although no chemical compound could explain it. As mentioned before, methyl salicylate has been identified to bring a fresh and minty aroma [77]. It would be interesting to dose this compound in wines made with stems in order to see if it could explain the increased “freshness”. It seems that the stems have a simultaneous action on several factors, so the result is an increase in complexity and freshness of the wines. More studies seem to be needed to understand the complex effect of stems on wine quality.
3.4.4. Wine Aging Potential and Stability
The impact of stem use on Tinta Miuda wine aging was studied by Sun and Spranger, who found that anthocyanin content was significantly affected after two years of bottle aging [89]. The anthocyanin content at bottling was higher for wines made without stems. It then decreased and reached a level where no significant difference could be detected between the two winemaking techniques. Individual anthocyanins were affected differently, where decreased delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidin-3-O-glucoside, peonidin-3-O-glucoside, and palvidin-6′′-O-acetylglucoside concentrations were not significantly affected by the presence of stems. Conversely, when stems where used, palvidin-3-O-glucoside and palvidine-3-O-glucoside-pyruvic acid adducts decreased more, whereas decreases in palvidine-6′′-O-p-coumarylglucoside and peonidine-6′′-O-p-coumarylglucoside were less important. The flavan-3-ols and the proanthocyanidins suggested that procyanidin B2, B3, B4, and C1 decreases were significantly lower when stems were used. Total oligomeric and polymeric proanthocyanidin contents showed lower decreases when stems were used. These results suggest that using stems during winemaking allows for a similar stability of anthocyanins as the control wine, but provides better stability of flavan-3-ols and proanthocyanidins. After two years of aging, mDP was significantly lowered but no significant differences were found between the stem and non-stem contact wine; the percentage of galloylation was not affected by the winemaking process or aging time; the total polyphenolic content stayed stable, suggesting that polyphenolic compounds were converted to other phenolic forms. No significant differences were found in wine color intensity, but the color of stem contact wine appeared more yellowish orange than the one of the non-stem contact wine.
Suriano et al. reported similar results regarding the anthocyanins content in Primitivo wines [81]. Fully destemmed grape wines showed higher anthocyanin content after 12 months of aging. For 25 to 50% of non-destemmed grape wines, color intensity increased, suggesting condensation phenomena between anthocyanins and tannins. No difference was found in the color shade after 12 months of aging, suggesting that using the whole harvest with stems could improve the color stability of wines during aging.
In Casassa et al.’s study, the Pinot Noir wines showed similar aging behavior after three and 12 months, regardless of the winemaking technique (added stems or 20% whole cluster) [84]; the anthocyanin content decreased, polymeric pigment levels increased, and tannin contents were stable. Same results were obtained for wine obtained using 50%, 100% whole cluster or dried stem [83]. Few studies have examined the impact of using stems on the aging potential. Excluding the rearrangement of anthocyanins classically observed in red wines, few conclusions can be drawn from these works. Although the antioxidant activity of stem extracts has been widely studied, the parallel with winemaking has not yet been sufficiently examined. If adding stems represents a source of antioxidants, it could be a way to reduce SO2. Two articles on this topic were found in the literature [76,86]. Ruiz-Moreno et al. showed positive results using stem extracts as a SO2 alternative in model solution for both antioxidant and antimicrobial action. In the other study, performed on red wines, Esparza et al. highlighted that the use of stem extracts could be a promising strategy to reduce SO2 in wines, but it still needs some optimization. In addition, a recent study on the use of grape stem extracts for protein precipitation showed that, in a model wine solution, these extracts could represent a good agent to remove unstable proteins [39]. Among the different grape varieties tested, Chasselas stem extracts, rich in polyphenols, showed the best results. Although used almost exclusively in red wines, it would be interesting to investigate the influence of stems on the protein stability of white wines.
Table 23 summarizes the main effects of the use of stems or whole clusters on wines from an oenological point of view.
Table 23.
Main effect of the use of stems or whole clusters on wines from an oenological point of view.
| Parameter | Variation | Percentage of Change Compared to Fully Destemmed Wines | References |
|---|---|---|---|
| pH | (+) | 1 to 9 | [3,12,79,83,84] |
| 0 | [3,79,80,81,84] | ||
| Titrable acitidy | (−) | 2 to 15 | [12,83,84] |
| 0 | [79,83,84] | ||
| Volatile acidity | (+) | 4 to 44 | [83,84] |
| (−) | 6 to 12 | [81,84] | |
| 0 | [79,83,84] | ||
| Potassium (K) | (+) | 2 to 33 | [3,80] |
| Ethanol content | (+) | 1 to 3 | [81,83] |
| (−) | 1 to 8 | [12,80,81,83,84] | |
| 0 | [79,83] | ||
| Total polyphenolic content | (+) | 14 to 30 | [3,12,80,81] |
| 0 | [3,79] | ||
| Total proanthocyanidin content | (+) | 7 to 225 | [12,30,81,84] |
| (−) | 19 | [83,84] | |
| 0 | [84] | ||
| Total anthocyanin content | (+) | 7 to 12 | [30,84] |
| (−) | 1 to 22 | [12,30,79,80,81,83,84] | |
| 0 | [81,83] | ||
| Color Intensity | (+) | 7 to 33 | [81] |
| (−) | 1 to 26 | [12,30] | |
| 0 | [83,84] | ||
| Color hue | (+) | 10 to 17 | [3,12] |
| (−) | 3 to 7 | [81] | |
| 0 | [30] | ||
| Aroma and volatile compounds | (+) | 1-Hexanol, IPMP, SBMP, IBMP | [73,79,82,84,85] |
| Taste | (+) | astringency and bitterness | [12,83,84] |
| (+) | complexity and freshness | [83] |
4. Conclusions
Analysis of the available research has allowed us to highlight the main compounds that compose stems. Although they do not seem to contain any new specific compounds, the transfer of certain molecules such as metal ions, phenolic compounds, or even some aromatic compounds, may induce changes in equilibrium, and thus could explain the increase in aromatic complexity in some cases. Stems’ high phenolic compound content could make them good candidates for antioxidants and stabilizers. Stem composition was mainly studied to evaluate their potential use as a source of compounds of interest, particularly phenolic compounds and stilbenes, for other sectors, such as pharmacy and health. Consequently, stem extracts are often obtained through extraction procedures that involve treating the stems upstream (freezing, grounding, etc.) using strong organic solvents to produce good yields. This does not represent the extraction phenomena that could take place during the winemaking process. It would be interesting to approach the extraction procedures in a similar way to alcoholic fermentation and maceration processes. This would identify which stem components have a real impact on the must and the wine matrices.
For winemaking trials with stems, the variability of grape varieties and limited knowledge regarding stem maturity makes it difficult to compare the different studies. However, several points emerged, such as decreased alcohol content, increased pH, and decreased anthocyanins content. Very few studies investigated the impact of stems on the stability and aging potential of wines. It would be interesting to look further into the phenolic compounds present in the stem extracts and their antioxidant capacity.
In using whole bunches of grapes or only stems, it is important to consider the general state of the stems. Very few studies focused on stem maturity or the degree of lignification, which varies according to the grape variety, the terroirs, and the vintage. Using stems is not systematic for a winegrower; it depends on the conditions of the vintage. Therefore, it is appropriate to consider the state of the stems to acquire new knowledge and facilitate a better understanding of this winemaking technique.
Acknowledgments
The authors would like to thank the viticulture and the wine technology and analysis teams for their collaboration. The authors also wish to acknowledge and thank Alain Handley for his translation and editorial assistance.
Abbreviations
| ABTS | 2,2-azinobis(3-ethylbenzthiazoline-6-sulphonic acid) |
| B1 (2, 3, 4 etc.) | Procyanidin dimer B1 (2, 3, 4 etc.) |
| CAE | Catechin equivalent |
| Cat | Catechin |
| CUPRAC | Cupric reducing antioxidant capacity |
| CyE | Cyanidin equivalent |
| DM | Dry matter |
| DMACA | 4-(N,N′-dimethylamino)cinnamaldehyde |
| DPPH | 1,1-diphenyl-2-picrylhydrazine |
| Ec | Epicatechin |
| EcG | Epicatechin gallate |
| Egc | Epigallocatechin |
| EgcG | Epigallocatechin gallate |
| ESI | Electron spray I |
| FI | Folin Index |
| FRAP | Ferric reducing antioxidant power |
| FW | Fresh weight |
| GAE | Gallic acid equivalent |
| Gc | Gallocatechin |
| GC—MS | Gas Chromatography—mass spectrometry |
| HPLC | High pressure liquid chromatography |
| IBMP | 2-methoxy-3-isobutylpyrazine |
| ICP | Inductive coupled plasma |
| IPMP | 2-methoxy-3-isopropylpyrazine |
| LCMS | Liquid chromatography—mass spectrometry |
| MC | Methyl cellulose |
| mDP | Mean degree of polymerization |
| ORAC | Oxygen radical absorbance capacity |
| OZ | Odorant zone |
| SBMP | 2-methoxy-3-sec-butylpyrazine |
| TPI | Total polyphenolic index |
Author Contributions
Conceptualization, B.B., L.Z., M.C. and M.B.; writing—original draft preparation, M.C., Á.D.-N. and M.B.; writing—review and editing, M.B. and Á.D.-N.; supervision, G.B., F.L. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ribéreau-Gayon P., Dubourdieu D., Donèche B., Lonvaud A. Traité d’oenologie: Microbiologie du vin, Vinifications. 7th ed. Volume 1 Dunod; Paris, France: 2017. [Google Scholar]
- 2.Foulonneau C. La Vinification. 4th ed. Dunod; Paris, France: 2014. [Google Scholar]
- 3.Hashizume K., Kida S., Samuta T. Effect of Steam Treatment of Grape Cluster Stems on the Methoxypyrazine, Phenolic, Acid, and Mineral Content of Red Wines Fermented with Stems. J. Agric. Food Chem. 1998;46:4382–4386. doi: 10.1021/jf9801771. [DOI] [Google Scholar]
- 4.Bioteau C. Davantage de fraîcheur avec les rafles. Réussir Vigne. [(accessed on 15 December 2020)];2017 Sep;:20–21. Available online: http://www.vitisphere.com/images/magazine/Rafles_Bordeaux.pdf.
- 5.Bazireau M. Vinification, Des rafles dans les bordeaux. La Vigne. [(accessed on 15 December 2020)];2016 Oct;:52–53. Available online: https://renadocagri.fr/index.php?lvl=notice_display&id=190497.
- 6.Vázquez-Armenta F.J., Silva-Espinoza B.A., Cruz-Valenzuela M.R., González-Aguilar G.A., Nazzaro F., Fratianni F., Ayala-Zavala J.F. Antibacterial and antioxidant properties of grape stem extract applied as disinfectant in fresh leafy vegetables. J. Food Sci. Technol. 2017;54:3192–3200. doi: 10.1007/s13197-017-2759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva V., Igrejas G., Falco V., Santos T.P., Torres C., Oliveira A.M., Pereira J.E., Amaral J.S., Poeta P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control. 2018;92:516–522. doi: 10.1016/j.foodcont.2018.05.031. [DOI] [Google Scholar]
- 8.Anastasiadi M., Chorianopoulos N.G., Nychas G.-J.E., Haroutounian S.A. Antilisterial Activities of Polyphenol-Rich Extracts of Grapes and Vinification Byproducts. J. Agric. Food Chem. 2009;57:457–463. doi: 10.1021/jf8024979. [DOI] [PubMed] [Google Scholar]
- 9.Apostolou A., Stagos D., Galitsiou E., Spyrou A., Haroutounian S., Portesis N., Trizoglou I., Hayes A.W., Tsatsakis A.M., Kouretas D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013;61:60–68. doi: 10.1016/j.fct.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Goutzourelas N., Stagos D., Spanidis Y., Liosi M., Apostolou A., Priftis A., Haroutounian S., Spandidos D.A., Tsatsakis A.M., Kouretas D. Polyphenolic composition of grape stem extracts affects antioxidant activity in endothelial and muscle cells. Mol. Med. Rep. 2015;12:5846–5856. doi: 10.3892/mmr.2015.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tello J., Ibáñez Marcos J. Evaluation of indexes for the quantitative and objective estimation of grapevine bunch compactness. Vitis J. Grapevine Res. 2014;53:9–16. [Google Scholar]
- 12.Pascual O., González-Royo E., Gil M., Gómez-Alonso S., García-Romero E., Canals J.M., Hermosín-Gutíerrez I., Zamora F. Influence of Grape Seeds and Stems on Wine Composition and Astringency. J. Agric. Food Chem. 2016;64:6555–6566. doi: 10.1021/acs.jafc.6b01806. [DOI] [PubMed] [Google Scholar]
- 13.González-Centeno M.R., Rosselló C., Simal S., Garau M.C., López F., Femenia A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT Food Sci. Technol. 2010;43:1580–1586. doi: 10.1016/j.lwt.2010.06.024. [DOI] [Google Scholar]
- 14.Rice A.C. Solid Waste Generation and By-Product Recovery Potential from Winery Residues. Am. J. Enol. Vitic. 1976;27:21–26. [Google Scholar]
- 15.McKendry P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002;83:37–46. doi: 10.1016/S0960-8524(01)00118-3. [DOI] [PubMed] [Google Scholar]
- 16.Ping L., Brosse N., Sannigrahi P., Ragauskas A. Evaluation of grape stalks as a bioresource. Ind. Crop. Prod. 2011;33:200–204. doi: 10.1016/j.indcrop.2010.10.009. [DOI] [Google Scholar]
- 17.Spigno G., Pizzorno T., De Faveri D.M. Cellulose and hemicelluloses recovery from grape stalks. Bioresour. Technol. 2008;99:4329–4337. doi: 10.1016/j.biortech.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Prozil S.O., Evtuguin D.V., Lopes L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crop. Prod. 2012;35:178–184. doi: 10.1016/j.indcrop.2011.06.035. [DOI] [Google Scholar]
- 19.Cruz J.M., Domínguez H., Parajó J.C. Assessment of the Production of Antioxidants from Winemaking Waste Solids. J. Agric. Food Chem. 2004;52:5612–5620. doi: 10.1021/jf049376c. [DOI] [PubMed] [Google Scholar]
- 20.Amendola D., De Faveri D., Egües I., Serrano L., Labidi J., Spigno G. Autohydrolysis and organosolv process for recovery of hemicelluloses, phenolic compounds and lignin from grape stalks. Bioresour. Technol. 2012;107:267–274. doi: 10.1016/j.biortech.2011.12.108. [DOI] [PubMed] [Google Scholar]
- 21.Llobera A., Cañellas J. Antioxidant activity and dietary fibre of Prensal Blanc white grape (Vitis vinifera) by-products. Int. J. Food Sci. Technol. 2008;43:1953–1959. doi: 10.1111/j.1365-2621.2008.01798.x. [DOI] [Google Scholar]
- 22.Ossorio P., Ballesteros Torres P. Chapter 17—Sensory Analysis of Red Wines for Winemaking Purposes. In: Morata A., editor. Red Wine Technology. Academic Press; Cambridge, MA, USA: 2019. pp. 253–256. [Google Scholar]
- 23.Llobera A., Cañellas J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007;101:659–666. doi: 10.1016/j.foodchem.2006.02.025. [DOI] [Google Scholar]
- 24.Rodríguez R. Master’s Thesis. University of Vigo; Vigo, Spain: 1999. Utilización de Residuos de la Industria Vitivinícola Para su Aprovechamiento. Composición del Bagazo de Cv. Albariño. [Google Scholar]
- 25.Moreira M.M., Barroso M.F., Porto J.V., Ramalhosa M., Švarc-Gajić J., Estevinho L., Morais S., Delerue-Matos C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total. Environ. 2018;634:831–842. doi: 10.1016/j.scitotenv.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Luque-Rodríguez J.M., Pérez-Juan P., Luque de Castro M.D. Extraction of Polyphenols from Vine Shoots of Vitis vinifera by Superheated Ethanol−Water Mixtures. J. Agric. Food Chem. 2006;54:8775–8781. doi: 10.1021/jf061855j. [DOI] [PubMed] [Google Scholar]
- 27.Rajha H.N., Boussetta N., Louka N., Maroun R.G., Vorobiev E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014;65:462–468. doi: 10.1016/j.foodres.2014.04.024. [DOI] [Google Scholar]
- 28.Alonso Á.M., Guillén D.A., Barroso C.G., Puertas B., García A. Determination of Antioxidant Activity of Wine Byproducts and Its Correlation with Polyphenolic Content. J. Agric. Food Chem. 2002;50:5832–5836. doi: 10.1021/jf025683b. [DOI] [PubMed] [Google Scholar]
- 29.Anastasiadi M., Pratsinis H., Kletsas D., Skaltsounis A.-L., Haroutounian S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT. 2012;48:316–322. doi: 10.1016/j.lwt.2012.04.006. [DOI] [Google Scholar]
- 30.Ribéreau-Gayon P., Mihlé J.C. Recherches technologiques sur les compositions phénoliques des vins rouges: Influence des différentes parties de la grappe. J. Int. Sci. Vigne Vin. 1977;11 doi: 10.20870/oeno-one.1970.4.1.1977. [DOI] [Google Scholar]
- 31.González-Centeno M.R., Jourdes M., Femenia A., Simal S., Rosselló C., Teissedre P.-L. Proanthocyanidin Composition and Antioxidant Potential of the Stem Winemaking Byproducts from 10 Different Grape Varieties (Vitis vinifera L.) J. Agric. Food Chem. 2012;60:11850–11858. doi: 10.1021/jf303047k. [DOI] [PubMed] [Google Scholar]
- 32.Kantz K., Singleton V.L. Isolation and Determination of Polymeric Polyphenols Using Sephadex LH-20 and Analysis of Grape Tissue Extracts. Am. J. Enol. Vitic. 1990;41:223–228. [Google Scholar]
- 33.Gouvinhas I., Pinto R., Santos R., Saavedra M.J., Barros A.I. Enhanced phytochemical composition and biological activities of grape (Vitis vinifera L.) Stems growing in low altitude regions. Sci. Hortic. 2020;265:109248. doi: 10.1016/j.scienta.2020.109248. [DOI] [Google Scholar]
- 34.Spatafora C., Barbagallo E., Amico V., Tringali C. Grape stems from Sicilian Vitis vinifera cultivars as a source of polyphenol-enriched fractions with enhanced antioxidant activity. LWT. 2013;54:542–548. doi: 10.1016/j.lwt.2013.06.007. [DOI] [Google Scholar]
- 35.Makris D.P., Boskou G., Andrikopoulos N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2007;98:2963–2967. doi: 10.1016/j.biortech.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Teixeira N., Mateus N., De Freitas V., Oliveira J. Wine industry by-product: Full polyphenolic characterization of grape stalks. Food Chem. 2018;268:110–117. doi: 10.1016/j.foodchem.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 37.Karvela E., Makris D.P., Kalogeropoulos N., Karathanos V.T. Deployment of response surface methodology to optimise recovery of grape (Vitis vinifera) stem polyphenols. Talanta. 2009;79:1311–1321. doi: 10.1016/j.talanta.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 38.Jiménez-Moreno N., Volpe F., Moler J.A., Esparza I., Ancín-Azpilicueta C. Impact of Extraction Conditions on the Phenolic Composition and Antioxidant Capacity of Grape Stem Extracts. Antioxidants. 2019;8:597. doi: 10.3390/antiox8120597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosińska-Cagnazzo A., Heeger A., Udrisard I., Mathieu M., Bach B., Andlauer W. Phenolic compounds of grape stems and their capacity to precipitate proteins from model wine. J. Food Sci. Technol. 2019;57:435–443. doi: 10.1007/s13197-019-04071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souquet J.-M., Labarbe B., Le Guernevé C., Cheynier V., Moutounet M. Phenolic Composition of Grape Stems. J. Agric. Food Chem. 2000;48:1076–1080. doi: 10.1021/jf991171u. [DOI] [PubMed] [Google Scholar]
- 41.Püssa T., Floren J., Kuldkepp P., Raal A. Survey of GrapevineVitis viniferaStem Polyphenols by Liquid Chromatography−Diode Array Detection−Tandem Mass Spectrometry. J. Agric. Food Chem. 2006;54:7488–7494. doi: 10.1021/jf061155e. [DOI] [PubMed] [Google Scholar]
- 42.Marchante L., Loarce L., Izquierdo-Cañas P.M., Alañón M.E., García-Romero E., Pérez-Coello M.S., Díaz-Maroto M.C. Natural extracts from grape seed and stem by-products in combination with colloidal silver as alternative preservatives to SO2 for white wines: Effects on chemical composition and sensorial properties. Food Res. Int. 2019;125:108594. doi: 10.1016/j.foodres.2019.108594. [DOI] [PubMed] [Google Scholar]
- 43.Del Llaudy M.C., Canals R., Canals J.M., Zamora F. Influence of ripening stage and maceration length on the contribution of grape skins, seeds and stems to phenolic composition and astringency in wine-simulated macerations. Eur. Food Res. Technol. 2007;226:337–344. doi: 10.1007/s00217-006-0542-3. [DOI] [Google Scholar]
- 44.Leal C., Santos R.A., Pinto R., Queiroz M., Rodrigues M., Saavedra M.J., Barros A., Gouvinhas I. Recovery of bioactive compounds from white grape (Vitis vinifera L.) stems as potential antimicrobial agents for human health. Saudi J. Biol. Sci. 2020;27:1009–1015. doi: 10.1016/j.sjbs.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Llobera A. Study on the antioxidant activity of grape stems (Vitis vinifera). A preliminary assessment of crude extracts. Food Nutr. Sci. 2012;3:500–504. [Google Scholar]
- 46.Barros A., Gouvinhas I., Machado N., Pinto J., Cunha M., Rosa E., Domínguez-Perles R. New grape stems-based liqueur: Physicochemical and phytochemical evaluation. Food Chem. 2016;190:896–903. doi: 10.1016/j.foodchem.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 47.Barros A., Girones-Vilaplana A., Teixeira A., Collado-González J., Moreno D.A., Gil-Izquierdo A., Rosa E., Domínguez-Perles R. Evaluation of grape (Vitis vinifera L.) stems from Portuguese varieties as a resource of (poly)phenolic compounds: A comparative study. Food Res. Int. 2014;65:375–384. doi: 10.1016/j.foodres.2014.07.021. [DOI] [Google Scholar]
- 48.Leal C., Gouvinhas I., Santos R.A., Rosa E., Silva A.M., Saavedra M.J., Barros A.I. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crop. Prod. 2020;154:112675. doi: 10.1016/j.indcrop.2020.112675. [DOI] [Google Scholar]
- 49.Makris D.P., Boskou G., Andrikopoulos N.K., Kefalas P. Characterisation of certain major polyphenolic antioxidants in grape (Vitis vinifera cv. Roditis) stems by liquid chromatography-mass spectrometry. Eur. Food Res. Technol. 2008;226:1075–1079. doi: 10.1007/s00217-007-0633-9. [DOI] [Google Scholar]
- 50.Ribéreau-Gayon P., Dubourdieu D., Glories Y., Maujean A. Traité d’Œnologie: Chimie du Vin, Stabilisation et Traitements. 7th ed. Volume 2 Dunod; Paris, France: 2017. [Google Scholar]
- 51.Bavaresco L., Cantu E., Fregoni M., Trevisan M. Constitutive stilbene contents of grapevine cluster stems as potential source of resveratrol in wine. VITIS J. Grapevine Res. 1997;36:115. [Google Scholar]
- 52.Piñeiro Z., Guerrero R.F., Fernández-Marin M.I., Cantos-Villar E., Palma M. Ultrasound-Assisted Extraction of Stilbenoids from Grape Stems. J. Agric. Food Chem. 2013;61:12549–12556. doi: 10.1021/jf4030129. [DOI] [PubMed] [Google Scholar]
- 53.Dias C., Domínguez-Perles R., Aires A., Teixeira A., Rosa E.A.S., Barros A., Saavedra M.J. Phytochemistry and activity against digestive pathogens of grape (Vitis vinifera L.) stem’s (poly)phenolic extracts. LWT. 2015;61:25–32. doi: 10.1016/j.lwt.2014.11.033. [DOI] [Google Scholar]
- 54.Rothwell J.A., Day A.J., Morgan M.R.A. Experimental Determination of Octanol−Water Partition Coefficients of Quercetin and Related Flavonoids. J. Agric. Food Chem. 2005;53:4355–4360. doi: 10.1021/jf0483669. [DOI] [PubMed] [Google Scholar]
- 55.Vivas N., Nonier M.-F., de Gaulejac N.V., Absalon C., Bertrand A., Mirabel M. Differentiation of proanthocyanidin tannins from seeds, skins and stems of grapes (Vitis vinifera) and heartwood of Quebracho (Schinopsis balansae) by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and thioacidolysis/liquid chromatography/electrospray ionization mass spectrometry. Anal. Chim. Acta. 2004;513:247–256. [Google Scholar]
- 56.Jordão A.M., Ricardo da Silva J.M., Laureano O. Evolution of proanthocyanidins in bunch stems during berry development (Vitis vinifera L.) VITIS Geilweilerhof. 2001;40:17–22. [Google Scholar]
- 57.Sun B.S., Pinto T., Leandro M.C., Ricardo-Da-Silva J.M., Spranger M.I. Transfer of Catechins and Proanthocyanidins From Solid Parts of the Grape Cluster Into Wine. Am. J. Enol. Vitic. 1999;50:179–184. [Google Scholar]
- 58.Guerra M.T., Santos-Buelga C., Escribano-Bailon M.T. Proanthocyanidins in skins from different grape varieties. Eur. Food Res. Technol. 1995;200:221–224. doi: 10.1007/bf01190499. [DOI] [Google Scholar]
- 59.Jordão A.M., Ricardo-da-Silva J.M., Laureano O. Evolution of Catechins and Oligomeric Procyanidins during grape maturation of Castelão Francês and Touriga Francesa. Am. J. Enol. Vitic. 2001;52:230–234. [Google Scholar]
- 60.González-Manzano S., Rivas-Gonzalo J.C., Santos-Buelga C. Extraction of flavan-3-ols from grape seed and skin into wine using simulated maceration. Anal. Chim. Acta. 2004;513:283–289. doi: 10.1016/j.aca.2003.10.019. [DOI] [Google Scholar]
- 61.Bourzeix M., Weyland D., Heredia N., Desfeux C. Etude des catéchines et des procyanidols de la grappe de raisin, du vin et d’autres dérivés de la vigne. Bull. l’OIV. 1986;59:1171–1254. [Google Scholar]
- 62.Da Silva J.M.R., Rigaud J., Cheynier V., Cheminat A., Moutounet M. Procyanidin dimers and trimers from grape seeds. Phytochemistry. 1991;30:1259–1264. doi: 10.1016/S0031-9422(00)95213-0. [DOI] [Google Scholar]
- 63.Porter L.J., Hrstich L.N., Chan B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry. 1985;25:223–230. doi: 10.1016/S0031-9422(00)94533-3. [DOI] [Google Scholar]
- 64.Harbertson J.F., Kennedy J.A., Adams D.O. Tannin in Skins and Seeds of Cabernet Sauvignon, Syrah, and Pinot noir Berries during Ripening. Am. J. Enol. Vitic. 2002;53:54–59. [Google Scholar]
- 65.Li L., Sun B. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2019;59:563–579. doi: 10.1080/10408398.2017.1381071. [DOI] [PubMed] [Google Scholar]
- 66.Chira K., Jourdes M., Teissedre P.-L. Cabernet sauvignon red wine astringency quality control by tannin characterization and polymerization during storage. Eur. Food Res. Technol. 2012;234:253–261. doi: 10.1007/s00217-011-1627-1. [DOI] [Google Scholar]
- 67.El Rayess Y., Barbar R., Wilson E.A., Bouajila J. Analytical Methods for Wine Polyphenols Analysis and for Their Antioxidant Activity Evaluation. Nova Science Publishers; New York, NY, USA: 2014. pp. 71–101. [Google Scholar]
- 68.Dudonné S., Vitrac X., Coutière P., Woillez M., Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 69.Rockenbach I.I., Rodrigues E., Gonzaga L.V., Caliari V., Genovese M.I., Gonçalves A.E.D.S.S., Fett R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011;127:174–179. doi: 10.1016/j.foodchem.2010.12.137. [DOI] [Google Scholar]
- 70.Pulido R.G., Hernández-García M., Sauracalixto F. Contribution of beverages to the intake of lipophilic and hydrophilic antioxidants in the Spanish diet. Eur. J. Clin. Nutr. 2003;57:1275–1282. doi: 10.1038/sj.ejcn.1601685. [DOI] [PubMed] [Google Scholar]
- 71.Garaguso I., Nardini M. Polyphenols content, phenolics profile and antioxidant activity of organic red wines produced without sulfur dioxide/sulfites addition in comparison to conventional red wines. Food Chem. 2015;179:336–342. doi: 10.1016/j.foodchem.2015.01.144. [DOI] [PubMed] [Google Scholar]
- 72.Nardini M., Garaguso I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020;305:125437. doi: 10.1016/j.foodchem.2019.125437. [DOI] [PubMed] [Google Scholar]
- 73.Hashizume K., Samuta T. Green Odorants of Grape Cluster Stem and Their Ability To Cause a Wine Stemmy Flavor. J. Agric. Food Chem. 1997;45:1333–1337. doi: 10.1021/jf960635a. [DOI] [Google Scholar]
- 74.Roujou de Boubée D., Cumsille A.M., Pons M., Dubourdieu D. Location of 2-Methoxy-3-isobutylpyrazine in Cabernet Sauvignon Grape Bunches and Its Extractability during Vinification. Am. J. Enol. Vitic. 2002;53:1–5. [Google Scholar]
- 75.Matarese F., Cuzzola A., Scalabrelli G., D’Onofrio C. Expression of terpene synthase genes associated with the formation of volatiles in different organs of Vitis vinifera. Phytochemistry. 2014;105:12–24. doi: 10.1016/j.phytochem.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Ruiz-Moreno M.J., Raposo R., Cayuela J.M., Zafrilla P., Piñeiro Z., Moreno-Rojas J.M., Mulero J., Puertas B., Giron F., Guerrero R.F., et al. Valorization of grape stems. Ind. Crop. Prod. 2015;63:152–157. doi: 10.1016/j.indcrop.2014.10.016. [DOI] [Google Scholar]
- 77.Poitou X. Master’s Thesis. Université Bordeaux; Bordeaux, France: 2016. Contribution to the aromatic knowledge of red wines: Sensory and molecular approach of the green nuances related to their origin. [Google Scholar]
- 78.Sahpazidou D., Geromichalos G.D., Stagos D., Apostolou A., Haroutounian S.A., Tsatsakis A.M., Tzanakakis G.N., Hayes A.W., Kouretas D. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicol. Lett. 2014;230:218–224. doi: 10.1016/j.toxlet.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 79.Spranger M.I., Clímaco M.C., Sun B., Eiriz N., Fortunato C., Nunes A., Leandro M.C., Avelar M.L., Belchior A.P. Differentiation of red winemaking technologies by phenolic and volatile composition. Anal. Chim. Acta. 2004;513:151–161. doi: 10.1016/j.aca.2004.01.023. [DOI] [Google Scholar]
- 80.Sun B.S., Spranger I., Roque-do-Vale F., Leandro C., Belchior P. Effect of Different Winemaking Technologies on Phenolic Composition in Tinta Miúda Red Wines. J. Agric. Food Chem. 2001;49:5809–5816. doi: 10.1021/jf010661v. [DOI] [PubMed] [Google Scholar]
- 81.Suriano S., Alba V., Tarricone L., Di Gennaro D. Maceration with stems contact fermentation: Effect on proanthocyanidins compounds and color in Primitivo red wines. Food Chem. 2015;177:382–389. doi: 10.1016/j.foodchem.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 82.Legrum C., Gracia-Moreno E., Lopez R., Potouridis T., Langen J., Slabizki P., Weiand J., Schmarr H.-G. Quantitative analysis of 3-alkyl-2-methoxypyrazines in German Sauvignon blanc wines by MDGC–MS or MDGC–MS/MS for viticultural and enological studies. Eur. Food Res. Technol. 2014;239:549–558. doi: 10.1007/s00217-014-2250-8. [DOI] [Google Scholar]
- 83.Casassa L.F., Dermutz N.P., Mawdsley P.F., Thompson M., Catania A.A., Collins T.S., Ashmore P.L., Du Fresne F., Gasic G., Peterson J.C.D. Whole Cluster and Dried Stem Additions’ Effects on Chemical and Sensory Properties of Pinot noir Wines over Two Vintages. Am. J. Enol. Vitic. 2021;72:21–35. doi: 10.5344/ajev.2020.20037. [DOI] [Google Scholar]
- 84.Casassa L.F., Sari S.E., Bolcato E.A., Diaz-Sambueza M.A., Catania A.A., Fanzone M.L., Raco F., Barda N. Chemical and Sensory Effects of Cold Soak, Whole Cluster Fermentation, and Stem Additions in Pinot noir Wines. Am. J. Enol. Vitic. 2018;70:19–33. doi: 10.5344/ajev.2018.18014. [DOI] [Google Scholar]
- 85.Benítez P., Castro R., Natera R., Barroso C.G. Effects of Grape Destemming on the Polyphenolic and Volatile Content of Fino Sherry Wine during Alcoholic Fermentation. Food Sci. Technol. Int. 2005;11:233–242. doi: 10.1177/1082013205056779. [DOI] [Google Scholar]
- 86.Esparza I., Martínez-Inda B., Cimminelli M.J., Jimeno-Mendoza M.C., Moler J.A., Jiménez-Moreno N., Ancín-Azpilicueta C. Reducing SO2 Doses in Red Wines by Using Grape Stem Extracts as Antioxidants. Biomolecules. 2020;10:1369. doi: 10.3390/biom10101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribéreau-Gayon J., Peynaud E., Sudraud P. Dosage des anthocyanes dans les vins rouges. Sci. Tech. Vin. 1972;1:497–499. [Google Scholar]
- 88.Ribéreau-Gayon P., Stonestreet E. Le dosage des anthocyanes dans le vin rouge. Bull. Soc. Chim. 1965;9:2649. [PubMed] [Google Scholar]
- 89.Sun B., Spranger M.I. Changes in phenolic composition of Tinta Miúda red wines after 2 years of ageing in bottle: Effect of winemaking technologies. Eur. Food Res. Technol. 2005;221:305–312. doi: 10.1007/s00217-005-1165-9. [DOI] [Google Scholar]