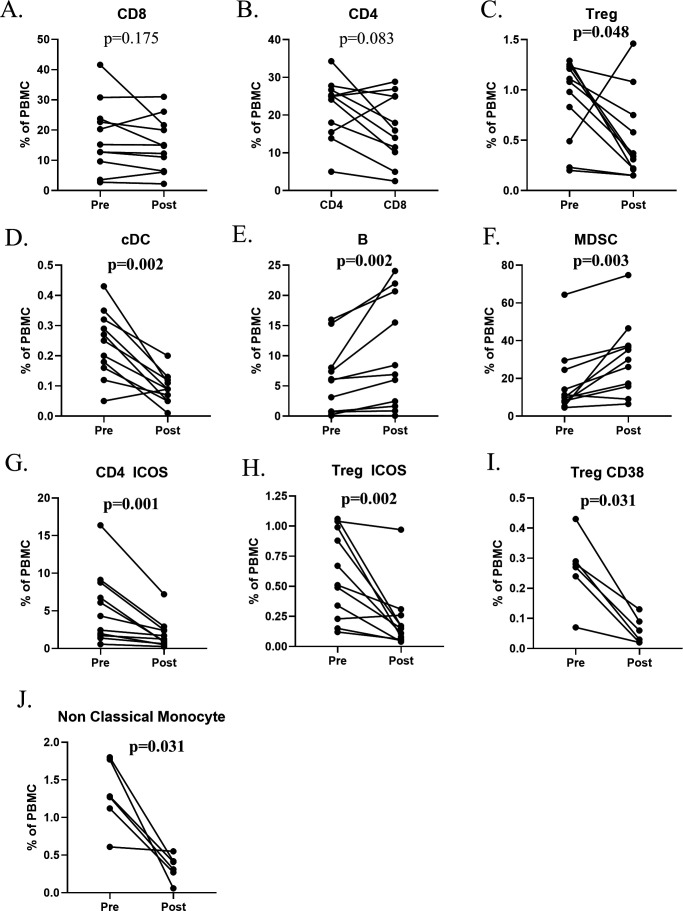
Figure 4.
Change in standard parental immune cell types and refined subsets after corticosteroids. Cancer patients (n=11) enrolled in immunotherapy trials received moderate- to high-dose corticosteroids (prednisone, n=6; methylprednisolone and prednisone, (n=4); or dexamethasone, methylprednisolone, and prednisone, n=1) for the development of immune-related adverse events. (A–F) Changes in standard parental immune cell types after corticosteroids. (G–J) Representative graphs are shown for notable refined subsets that changed with corticosteroids. Significant changes were defined by a p value <0.05, a median difference poststeroid versus presteroids >0.05% of PBMCs, and at least half of evaluated patients having a >25% change. The panels used for refined subsets reflecting maturation/functional status of subsets were slightly different for the various immunotherapy trials, so certain subsets were not tested in all patients (n=6 for Treg CD38 and non-classical monocytes). MDSCs, myeloid-derived suppressor cells; PBMCs, peripheral blood mononuclear cells; Tregs, regulatory T cells.