Abstract
Background: Cholinesterase inhibitors such as donepezil are used in the treatment of Alzheimer’s disease. Patients taking cholinesterase inhibitors can develop cholinergically mediated QT prolongation, which may lead to life-threatening arrhythmias. In this study we investigated the corrected QT interval (QTc) of patients taking donepezil.
Methods and Results: This study enrolled 114 outpatients attending Tarumizu Chuo Hospital. Subjects were divided into a donepezil group (n=57) or an age- and sex-matched control group (n=57). Physical findings, laboratory data, and electrocardiographic parameters were compared between the groups. QTc was significantly prolonged (mean [±SD] 0.443±0.032 s vs. 0.426±0.026s; P<0.001) and the percentage of patients with prolonged QTc was significantly higher (30% vs. 9%; P<0.01) in the donepezil than control group. Furthermore, in the donepezil group, QTc was significantly prolonged after patients started taking donepezil compared with baseline (from 0.433±0.034 to 0.442±0.033s; n=46; P<0.05). On univariate analysis, QTc was significantly associated with taking donepezil, as well as with hemoglobin, serum calcium concentration, and estimated glomerular filtration rate (eGFR; all P<0.01). On multivariate analysis, QTc was significantly associated with taking donepezil (P<0.001), serum potassium concentration (P<0.05), and eGFR (P<0.05).
Conclusions: The incidence of QTc prolongation was more frequent in patients taking donepezil than in the control group, and was difficult to predict. Periodic electrocardiogram examinations are recommended considering the possibility of adverse events, such as fatal arrhythmias.
Key Words: Adverse effect, Cholinesterase inhibitor, Donepezil, Fatal arrhythmias, QTc prolongation
There is an increasing trend in the overall incidence of dementia in Japan.1 Alzheimer’s disease is the most common cause of dementia affecting older people, and is characterized by the loss of cholinergic neurons in parts of the brain. Cholinesterase inhibitors such as donepezil, galantamine, and rivastigmine delay the breakdown of acetylcholine released into synaptic clefts and thus enhance cholinergic neurotransmission.2 These cholinesterase inhibitors are currently considered first-line agents in the treatment of Alzheimer’s disease.3
Although adverse cardiovascular events with these medicines are uncommon, there is evidence that cholinesterase inhibitor therapy is associated with a small but significant increase in the risk of syncope and bradycardia. There are also a few reports that these medicines may occasionally be associated with QTc prolongation and Torsades de Pointes (TdP) ventricular tachycardia.4 A small number of case reports suggest a possible association between donepezil and ventricular arrhythmias, in particular TdP.5–11 These reports suggest that donepezil may lead to prolongation of the QTc and predispose patients to development of TdP. The primary questions are whether cholinesterase inhibitors are associated with QTc prolongation and how many patients actually have prolonged QTc. Unfortunately, few studies have attempted to determine how many patients taking cholinesterase inhibitors have QTc prolongation. In this study we investigated QTc prolongation in patients taking the cholinesterase inhibitor donepezil and examined whether QTc prolongation could be predicted.
Methods
Study Subjects
In the present cross-sectional study we examined 57 consecutive outpatients >65 years of age who had Alzheimer’s disease, underwent treatment with donepezil, and had an electrocardiogram (ECG) examination as part of regular screening at Tarumizu Chuo Hospital between November 2013 and September 2017. In addition, 57 age- and sex-matched outpatients who had also visited Tarumizu Chuo Hospital during the same period of time for internal therapy without donepezil and had undergone an ECG examination were enrolled as the control group. Patients in the control group were not necessarily diagnosed with Alzheimer’s disease.
Exclusion criteria for both groups included the presence of atrial fibrillation, a history of cardiac surgery, and renal failure requiring hemodialysis. Cardiac surgery was defined as valvular disease surgery, coronary artery bypass surgery, congenital heart disease surgery, pacemaker implantation, and catheter ablation for arrhythmias. Patients taking antiarrhythmic medications, antibiotics, and first-generation antihistamines that affect QT prolongation were also excluded in both groups. Patients with comorbidities such as chronic heart failure, old myocardial infarction, hypertrophic cardiomyopathy, dilated cardiomyopathy, hypertensive heart disease, diabetes, and hypothyroidism were included in both groups. Chronic heart failure was defined as a history of hospitalization for heart failure or treatment following a diagnosis of heart failure.
This study was approved by the Ethics Committee of Tarumizu Chuo Hospital (No. 18-4). All study procedures were performed in accordance with the provisions of the Declaration of Helsinki, as revised in Brazil in 2013. Because of the retrospective and observational nature of the study, the requirement for written informed consent was waived, but patients who declined to participate in the study were excluded.
ECG Examination
All patients underwent a 12-lead ECG as part of regular screening during the study period. ECGs were recorded using an FCP-7431 (Fukuda Denshi, Tokyo, Japan) with a 25-mm/s paper speed and standardized at 0.1 mV/mm. ECG parameters, including heart rate, PR interval, QT interval, QTc, and QRS duration, were calculated automatically by the apparatus. The algorithm for automatic QT interval measurement by the differential threshold is based on the first derivative of the ECG waveform, as described below. The point at which the differential waveform of the T-wave returns to the background noise level is defined as the T-wave terminus, and its distance from the start of the QRS complex is measured. Kasamaki et al concluded that, for measurement of the QT/QTc interval, the differential threshold method appears to be suitable because of smaller inter-reader differences, better reproducibility, and similarity to visual measurements.12
The QTc was calculated for heart rate using Bazett’s formula (QTc = QT/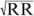 ).13
Prolonged QTc was defined as QTc >0.450 s in males and >0.460 s in females, according to the risk score of congenital long QT syndrome reported by Schwartz et al.14
In addition, in the donepezil group, QTc was compared before and after the start of donepezil treatment if the ECG had been recorded using the same method before the start of donepezil treatment.
).13
Prolonged QTc was defined as QTc >0.450 s in males and >0.460 s in females, according to the risk score of congenital long QT syndrome reported by Schwartz et al.14
In addition, in the donepezil group, QTc was compared before and after the start of donepezil treatment if the ECG had been recorded using the same method before the start of donepezil treatment.
Baseline Measurements and Biochemical Analyses
The day the ECG was performed, body height and weight were also measured, and concomitant medications and comorbidities were investigated. Body mass index (BMI) was calculated as weight in kilograms divided by height squared in meters (kg/m2). Blood samples were obtained from subjects in the fasting state. Plasma B-type natriuretic peptide (BNP) concentrations were determined using a chemiluminescent immunoassay method with an automatic analyzer (ADVIA Centaur CP; Siemens Healthcare, Tokyo, Japan). Serum creatinine concentrations, serum cholinesterase activity, and serum sodium, potassium, and calcium concentrations were determined according to standard laboratory procedures. Serum calcium concentrations were adjusted using the following formula of Payne et al:15
Adjusted calcium (mg/dL) = calcium (mg/dL) − albumin (g/dL) + 4.0
Estimated glomerular filtration rate (eGFR) was determined using the Modification of Diet in Renal Disease equation with coefficients modified for Japanese patients as follows:16
eGFR (mL/min/1.73 m2) = 194 × serum creatinine (mg/dL)−1.094 × age (years)−0.287 × (0.739 if female)
Statistical Analyses
Baseline characteristics are expressed as the number and percentage for nominal variables, as the mean±SD for normally distributed continuous variables, and as the median and interquartile range (IQR) for non-normally distributed continuous variables.
We compared physical findings, laboratory data, and electrocardiographic parameters between the 2 groups. According to the Guidelines for Diagnosis and Management of Inherited Arrhythmias published by the Japanese Circulation Society, advanced age, female sex, obesity, hypokalemia, hypocalcemia, chronic heart failure, and decreased renal function are risk factors for TdP in patients taking medications with a QTc-prolonging effect.17,18 In addition, anemia has been associated with QTc prolongation.19 Therefore, we investigated the relationship between QTc and these parameters.
Given 2 data sets, we assessed whether 2 samples of observations came from the same distribution. The Wilcoxon rank-sum test was used to identify significant differences between the 2 groups for variables with skewed and/or homoscedastic distributions. The proportions of comorbidities or patients taking particular medications were compared between the donepezil and control groups using the Chi-squared test. The percentage of patients with prolonged QTc was analyzed by logistic regression analysis. Comparison of QTc before and after the start of taking donepezil were analyzed using paired t-tests. The association between QTc and each parameter was analyzed using Pearson’s correlation coefficient. Univariate and multivariate linear regression analyses were used to assess predictors of QTc. All differences were considered significant at 2-tailed P<0.05. Statistical analyses were performed using JMP version 11.0 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
The clinical characteristics of the patients enrolled in the study are given in Table 1. There were no significant differences in age, sex, BMI, hemoglobin, plasma BNP concentration, serum cholinesterase activity, serum sodium or potassium concentration, or eGFR between the 2 groups. In the donepezil group, serum calcium concentration was significantly lower than in the control group, but the adjusted serum calcium concentration was not significantly different between the 2 groups. In addition, there were no significant differences between groups in the rate of concomitant medications possibly causing QT prolongation, such as histamine H2 receptor blockers and loop or thiazide diuretics. There were no significant differences between groups in the rate of comorbidities possibly causing QT prolongation, such as chronic heart failure, old myocardial infarction, hypertrophic cardiomyopathy, dilated cardiomyopathy, hypertensive heart disease, diabetes, and hypothyroidism. In the donepezil group, the daily donepezil doses were as follows: 3 mg in 2 subjects (4%), 5 mg in 51 subjects (89%), and 10 mg in 4 subjects (7%). The mean duration of donepezil exposure in the 50 patients with a traceable start date of medication was 528±376 days.
Table 1.
Patient Characteristics
| Donepezil (n=57) |
Control (n=57) |
P value | |
|---|---|---|---|
| Age (years) | 84.6±5.8 | 84.1±6.3 | 0.85 |
| Sex (no. males/females) | 21/36 | 21/36 | 1.00 |
| BMI (kg/m2) | 20.3±4.2 | 21.7±3.6 | 0.06 |
| Hemoglobin (g/dL) | 12.0±2.1 | 12.2±1.6 | 0.98 |
| BNP (pg/mL) | 72.5 [33.7–141.9] | 66.6 [47.0–146.5] | 0.53 |
| Serum cholinesterase activity (U/L) | 235±69 | 253±66 | 0.24 |
| Serum sodium (mEq/L) | 140±4.0 | 141±3.8 | 0.50 |
| Serum potassium (mEq/L) | 4.12±0.53 | 4.27±0.44 | 0.21 |
| Serum calcium (mg/dL) | 9.1±0.6 | 9.4±0.5 | 0.03 |
| Adjusted serum calcium (mg/dL) | 9.5±0.5 | 9.5±0.5 | 0.85 |
| eGFR (mL/min/1.73 m2) | 55.8±19.2 | 53.6±16.4 | 0.50 |
| Medications | |||
| Histamine H2 receptor blockers | 4 (7) | 4 (7) | 1.00 |
| Loop or thiazide diuretics | 10 (18) | 15 (26) | 0.26 |
| Comorbidities | |||
| Chronic heart failure | 13 (23) | 16 (28) | 0.52 |
| OMI | 5 (9) | 9 (16) | 0.25 |
| Hypertrophic cardiomyopathy | 0 (0) | 0 (0) | – |
| Dilated cardiomyopathy | 0 (0) | 0 (0) | – |
| Hypertensive heart disease | 0 (0) | 0 (0) | – |
| Diabetes | 21 (37) | 15 (26) | 0.23 |
| Hypothyroidism | 7 (12) | 14 (25) | 0.09 |
Unless indicated otherwise, data are given as n (%), the mean±SD, or median [interquartile range]. BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; OMI, old myocardial infarction.
Comparison of ECG Parameters Between the 2 Groups
Table 2 lists ECG parameters of the donepezil and control groups. There were no significant differences in heart rate, PR interval, or QRS duration. QTc was significantly prolonged in the donepezil compared with control group (0.443±0.032 s vs. 0.426±0.026s; P<0.001).
Table 2.
Electrocardiogram Parameters in the Donepezil and Control Groups
| Donepezil (n=57) |
Control (n=57) |
P value | |
|---|---|---|---|
| HR (beats/min) | 72±16 | 70±11 | 0.80 |
| PR (s) | 0.176±0.035 | 0.173±0.027 | 0.92 |
| QRS (s) | 0.105±0.019 | 0.099±0.012 | 0.18 |
| QTc (s) | 0.443±0.032 | 0.426±0.026 | <0.001 |
Values are the mean±SD. HR, heart rate.
Percentage of Patients With Prolonged QTc
The total percentage of patients with prolonged QTc was significantly higher in the donepezil than control group (30% vs. 9%; odds ratio 4.42, 95% confidence interval 1.50–13.0, P<0.01; Table 3). Among male patients, the percentage with QTc prolongation >0.450 s was 43% (n=9) in the donepezil group and 10% (n=2) in the control group. Among female patients, the percentage with QTc prolongation >0.460 s was 22% (n=8) in the donepezil group and 8% (n=3) in the control group.
Table 3.
Percentage of Patients With QTc Prolongation in the Donepezil and Control Groups
| Donepezil (n=57) |
Control (n=57) |
OR (95% CI) |
P value | |
|---|---|---|---|---|
| QTc prolongation | 17 (30) | 5 (9) | 4.42 (1.50–13.0) | 0.004 |
Unless indicated otherwise, data are given as n (%). QTc prolongation was defined as QTc >0.450 s in males and >0.460 s in females. CI, confidence interval; OR, odds ratio.
QTc Before and After the Start of Donepezil Treatment
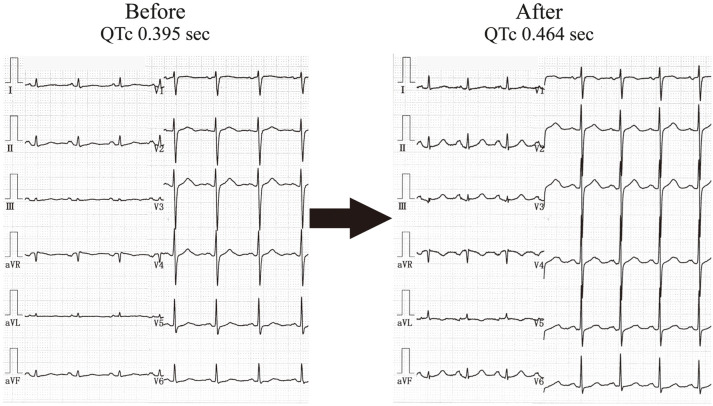
In 46 of the 57 patients in the donepezil group whose ECG was recorded before they started donepezil, the QTc after the start of donepezil treatment was significantly prolonged compared with before donepezil treatment (from 0.433±0.034 to 0.442±0.033s; P<0.05; Table 4). A typical case is shown in the Figure.
Table 4.
Changes in Mean (±SD) QTc Before and After the Start of Donepezil Treatment
| Before donepezil (n=46) |
After donepezil (n=46) |
P value | |
|---|---|---|---|
| QTc (s) | 0.433±0.034 | 0.442±0.033 | 0.014 |
Figure.
QTc in a patient before and after the start of donepezil. A 78-year-old female was taking donepezil 3 mg per day. The QTc measured 24 months before starting donepezil was 0.395 s, whereas 8 months after starting donepezil the QTc had increased to 0.464 s.
Relationship Between QTc and Patient Characteristics
The results of univariate and multivariate linear regression analyses for QTc are given in Table 5. On univariate analysis, QTc was associated with taking donepezil (β=0.008, P<0.01) hemoglobin (β=−0.004, P<0.01), serum calcium concentration (β=−0.020, P<0.01), and eGFR (β=−0.0004, P<0.01). On multivariate analysis, taking donepezil (β=0.014, P<0.001), serum potassium concentration (β=−0.017, P<0.05), and eGFR (β=−0.0006, P<0.05) were significantly associated with QTc.
Table 5.
Regression Analyses for QTc
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | t | P value | β | SE | t | P value | |
| Taking donepezil | 0.00835 | 0.00274 | 3.04 | 0.003 | 0.01412 | 0.00396 | 3.57 | <0.001 |
| Age | 0.00005 | 0.00048 | 0.10 | 0.92 | −0.00060 | 0.00068 | −0.88 | 0.39 |
| Female sex | −0.00204 | 0.00297 | −0.69 | 0.49 | −0.00467 | 0.00432 | −1.08 | 0.29 |
| BMI | −0.00043 | 0.00074 | −0.58 | 0.56 | 0.00083 | 0.00103 | 0.81 | 0.42 |
| Hemoglobin | −0.00425 | 0.00151 | −2.83 | 0.006 | −0.00498 | 0.00257 | −1.94 | 0.06 |
| Log [BNP] | 0.00542 | 0.00402 | 1.35 | 0.18 | 0.00317 | 0.00490 | 0.65 | 0.52 |
| Serum potassium | −0.00120 | 0.00580 | −0.21 | 0.84 | −0.01744 | 0.00766 | −2.28 | 0.03 |
| Serum calcium | −0.02008 | 0.00661 | −3.04 | 0.003 | −0.00477 | 0.00772 | −0.62 | 0.54 |
| eGFR | −0.00044 | 0.00016 | −2.84 | 0.005 | −0.00058 | 0.00023 | −2.52 | 0.02 |
BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; SE, standard error.
Discussion
Acquired long QT syndrome is characterized by QT prolongation and sometimes TdP ventricular tachycardia, and the condition can be provoked by QT-prolonging drugs. Agents associated with the development of acquired long QT syndrome include many in common use, such as antihistamines, antibiotics, and antidepressants, among others.18 Treatment with cholinesterase inhibitors may also result in life-threatening ventricular tachycardia in patients without overt coronary artery disease or biochemical abnormalities.10 Therefore, the package inserts of these medications state that their use carries a possibility of QT prolongation. Activation of cardiac acetylcholine receptors opens voltage-gated calcium channels, whereas inhibition of acetylcholinesterase may lead to increased intracellular calcium concentrations. This may, in turn, prolong Phase 2 of the cardiac action potential, thereby increasing the risk of ventricular arrhythmias.10
A summary of previous case reports of donepezil-induced fatal arrhythmias is provided in Table 6.5–11 In most cases, QTc prolongation caused arrhythmias. The duration of taking donepezil at the onset of the arrhythmic event varied from 1 month to 3 years. In 2 of these cases,7,10 the event occurred days after increasing the dose of donepezil from 5 to 10 mg. The number of concomitant medications other than donepezil also varied from 0 to 6. In all these case reports, discontinuation of donepezil improved QTc prolongation, and no recurrence of arrhythmias was reported. Even in the case of TdP without QTc prolongation,9 the arrhythmia did not recur with donepezil discontinuation. Based on these case reports and the results of the present study, it is difficult to predict the occurrence of QTc prolongation and arrhythmias in patients taking donepezil.
Table 6.
Summary of Previously Reported Cases Describing QTc and Fatal Arrhythmias in Patients Taking Donepezil
| Case report | Patient age (years), sex |
Chief complaint | Types of arrhythmia | Comorbidities | Daily dose of donepezil |
Concomitant medications (daily dose) |
QTc at first visit (s) |
Treatment | Outcome | QTc at discharge (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Suleyman et al5 | 82, M | Dizziness and syncope |
CAVB and NSVT | None | 10 mg for the previous 1 month |
None | Unknown | Temporary pacemaker and discontinuation of donepezil |
Discharged on Day 6 |
Unknown |
| Leitch et al6 | 76, F | Two syncope episodes |
Sinus bradycardia and TdP |
Depression | 10 mg for 2 years | Omeprazole 20 mg, escitalopram 10 mg, propranolol 80 mg |
0.590–0.777 | Intravenous magnesium, an infusion of isoprenaline, and temporary cardiac pacing and discontinuation of donepezil |
Discharged | 0.436 |
| Tanaka et al7 | ||||||||||
| Case 1 | 90, M | Accidental bradycardia |
Advanced atrioventricular block |
None | 10 mg (increased from 5 to 10 mg 3 days earlier) |
None | 0.514 (QT, not QTc) |
Donepezil was discontinued and orciprenaline was administered instead |
No significant arrhythmia occurred thereafter for 2 months |
0.456 |
| Case 2 | 87, F | Syncope, vomiting, and nausea |
TdP | HTN, AF | 5 mg | Cilostazol 100 mg, amlodipine 5 mg, spironolactone 25 mg, warfarin 1 mg |
0.594 | Orciprenaline was administered; donepezil was discontinued |
Discharged on Day 37 |
0.446 |
| Takaya et al8 | 83, F | Diarrhea, vomiting, and syncope |
TdP | HTN, DM, PAF, and OMI |
5 mg for at least 2 years |
Bisoprolol 5 mg | 0.645 | Discontinuation of donepezil |
Discharged on Day 14 |
0.485 |
| Hadano et al9 | 86, F | Syncope attack | TdP | HTN | 5 mg for 3 years | Amlodipine 5 mg | 0.436 | Intravenous infusion of lidocaine and discontinuation of donepezil |
Discharged | “Normal” |
| Kitt et al10 | 80, F | Diarrhea, vomiting, and worsening confusion |
Polymorphic ventricular tachycardia |
CVD, AF, pacemaker implantation |
10 mg (increased from 5 to 10 mg 2 weeks earlier) |
Bumetanide 2 mg, perindopril 8 mg, lansoprazole 30 mg, atorvastatin 20 mg, diltiazem 60 mg, fluoxetine 60 mg |
0.490 | Cardioversion, discontinuation of donepezil, and reduction of fluoxetine |
Discharged | 0.431 |
| Gurbuz et al11 | 84, F | Recurrent syncope | TdP | None | 10 mg for 1 year | None | 0.624 | Discontinuation of donepezil | No recurrence of syncope thereafter for 1 year |
0.430 |
AF, atrial fibrillation; CAVB, complete atrioventricular block; CVD, cerebrovascular disease; DM, diabetes mellitus; F, female; HTN, hypertension; M, male; NSVT, non-sustained ventricular tachycardia; OMI, old myocardial infarction; PAF, paroxysmal atrial fibrillation; TdP, Torsades de Pointes.
In this study, worsening eGFR was associated with QTc prolongation. The incidence of acquired long QT syndrome increases with declining kidney function in chronic kidney disease (CKD) patients.20 In the study of Sherif et al, two-thirds of patients with CKD had QTc interval prolongation, and approximately 20% had a QTc interval >500 ms.21 Significant QTc interval prolongation was associated with progression of CKD, independent of other risk factors such as age, sex, potassium concentration, and calcium concentration.21 The renin-angiotensin system (RAS) is thought to play a role in the mechanism of QTc prolongation in CKD patients. Because the RAS system is overactive in CKD, excess angiotensin II, the biologically active product of the RAS, accumulates in the heart, thereby promoting myocyte hypertrophy, fibroblast proliferation, interstitial accumulation of collagen, and microvascular disease. These myocardial structural changes are major determinants of an increase in myocardial stiffness, leading to left ventricular diastolic and systolic dysfunction and other complications, such as cardiac conduction disturbances and QTc prolongation.22 In addition, polymorphisms in the angiotensin-converting enzyme and angiotensin AT1 receptor genes additively contribute to QTc prolongation in a great majority of patients with end-stage CKD.23 In the present study, renal dysfunction was associated with QTc prolongation with or without donepezil treatment. Because both renal dysfunction and donepezil affect QTc prolongation, clinicians should carefully consider the possibility of adverse effects in CKD patients taking donepezil.
Several risk factors for TdP have been identified, including female sex, low potassium levels, hypomagnesemia, bradycardia, and older age.24,25 However, other recent studies indicate that drug-induced TdP can be associated with silent mutations and common polymorphisms in K+ channel genes responsible for congenital long QT syndrome.26 Genetic factors may underlie the susceptibility to drug-induced serious adverse reactions such as long QTc and TdP. When rapid component of the delayed rectifier K+ current (IKr)-blocking agents produce excessive QT prolongation, the underlying genetic background of the patient with drug-induced long QT syndrome should be taken into consideration, as would be the case with congenital long QT syndrome; drug-induced long QT syndrome can be regarded as a latent form of long QT syndrome.27 These reports confirm the unpredictability of drug-induced QTc prolongation.
It has been reported that approximately 3% of oral medications are capable of prolonging the QT interval.28 Furthermore, after initiation of a drug associated with TdP, ECG signs to look for that are indicative of a risk for arrhythmia include an increase in QTc from a pre-drug baseline of 0.060 s and marked QTc prolongation >0.500 s.29
In 2015, The Japan Geriatrics Society published the Guidelines for Medical Treatment and its Safety in the Elderly 2015.30 This guideline described the following criteria for considering the discontinuation of antidementia medications: (1) advanced Alzheimer’s disease patients no longer able to communicate or who have become bedridden or exhibit a worsened general condition; (2) the medications have become less effective; and (3) adverse events have occurred.30 In the present study, we determined the frequency of QTc prolongation in Alzheimer’s disease patients taking donepezil. Our results demonstrate that QTc prolongation may be associated with donepezil. Our results also suggest that regular QTc monitoring is needed in patients taking cholinesterase inhibitors. Caution is warranted with the use of these medications, because prolonged QTc may increase the risk of both arrhythmias and sudden death. When prescribing drugs, not only cholinesterase inhibitors, but also other drugs, clinicians should carefully consider whether the patient actually needs that drug. Drugs should be administered responsibly with sufficient consideration of potential adverse effects.
Study Limitations
Several limitations of this study must be considered when interpreting the results. First, the study population was relatively small; thus, the resulting statistical power may have been insufficient to demonstrate differences in some parameters. In addition, because this study was a retrospective analysis, data for some blood laboratory parameters were not available for all subjects. Therefore, BNP was measured in 34 patients, cholinesterase in 53, calcium in 39, and adjusted calcium in 28. Regardless, the present study does provide a preliminary framework for the planning of future prospective studies, such as multicenter investigations involving a larger numbers of cases. Second, we investigated only donepezil and did not examine other cholinesterase inhibitors, such as galantamine and rivastigmine. Moreover, we did not compare patients treated with donepezil based on the given dose (5 or 10 mg). This was because there were fewer patients taking medications other than donepezil and fewer patients taking donepezil at a dose other than 5 mg. Future studies should examine these patients as well. Third, QTc was calculated automatically, not manually. Donepezil is mostly prescribed by general clinicians (non-cardiologists); it may therefore be difficult for them to manually calculate the QTc. However, automatically calculated values are easy to check, even by general clinicians. Therefore, in this study, QTc was evaluated using automatic calculations. Fourth, we did not measure the blood concentration of donepezil in the present study. Finally, the control group patients were not necessarily diagnosed with Alzheimer’s disease. However, there are no reports indicating that Alzheimer’s disease per se causes prolonged QTc; thus, QTc prolongation in these patients was most likely associated with donepezil rather than the disease.
Conclusions
In patients taking donepezil, the incidence of QTc prolongation was higher than the control group but difficult to predict. This suggests that patients taking donepezil should undergo periodic ECG examinations considering the possibility of adverse effects with this drug.
Sources of Funding
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosures
M.O. is a member of Circulation Reports’ Editorial Team. The remaining authors report no potential conflicts of interest.
IRB Information
This study was approved by the Ethics Committee of Tarumizu Chuo Hospital (No. 18-4).
Acknowledgment
The authors thank Satoshi Abe, MD, PhD, former director of Tarumizu Chuo Hospital, for providing much constructive advice from the start of this study. Unfortunately, Dr. Abe died suddenly in 2019. The authors are grateful to him and sincerely pray for the repose of his soul.
References
- 1. Dodge HH, Buracchio TJ, Fisher GG, Kiyohara Y, Meguro K, Tanizaki Y, et al.. Trends in the prevalence of dementia in Japan. Int J Alzheimers Dis 2012; 2012: 956354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans JG, Wilcock G, Birks J.. Evidence-based pharmacotherapy of Alzheimer’s disease. Int J Neuropsychopharmacol 2004; 7: 351–369. [DOI] [PubMed] [Google Scholar]
- 3. Isik AT, Bozoglu E, Yay A, Soysal P, Ateskan U.. Which cholinesterase inhibitor is the safest for the heart in elderly patients with Alzheimer’s disease? Am J Alzheimers Dis Other Demen 2012; 27: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howes LG.. Cardiovascular effects of drugs used to treat Alzheimer’s disease. Drug Saf 2014; 37: 391–395. [DOI] [PubMed] [Google Scholar]
- 5. Suleyman T, Tevfik P, Abdulkadir G, Ozlem S.. Complete atrioventricular block and ventricular tachyarrhythmia associated with donepezil. Emerg Med J 2006; 23: 641–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leitch A, McGinness P, Wallbridge D.. Calculate the QT interval in patients taking drugs for dementia. BMJ 2007; 335: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanaka A, Koga S, Hiramatsu Y.. Donepezil-induced adverse side effects of cardiac rhythm: 2 cases report of atrioventricular block and Torsade de Pointes. Intern Med 2009; 48: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 8. Takaya T, Okamoto M, Yodoi K, Hata K, Kijima Y, Nakajima H, et al.. Torsades de Pointes with QT prolongation related to donepezil use. J Cardiol 2009; 54: 507–511. [DOI] [PubMed] [Google Scholar]
- 9. Hadano Y, Ogawa H, Wakeyama T, Iwami T, Kimura M, Mochizuki M, et al.. Donepezil-induced torsades de pointes without QT prolongation. J Cardiol Cases 2013; 8: e69–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitt J, Irons R, Al-Obaidi M, Missouris C.. A case of donepezil-related torsades de pointes. BMJ Case Rep 2015; 2015: bcr2015211900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gurbuz AS, Ozturk S, Acar E, Efe SC, Akgun T, Kilicgedik A, et al.. Acquired long QT syndrome and Torsades de Pointes related to donepezil use in a patient with Alzheimer disease. Egypt Heart J 2016; 68: 197–199. [Google Scholar]
- 12. Kasamaki Y, Ozawa Y, Ohta M, Sezai A, Yamaki T, Kaneko M, et al.. Automated versus manual measurement of the QT interval and corrected QT interval. Ann Noninvasive Electrocardiol 2011; 16: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roguin A.. Henry Cuthbert Bazett (1885–1950): The man behind the QT interval correction formula. Pacing Clin Electrophysiol 2011; 34: 384–388. [DOI] [PubMed] [Google Scholar]
- 14. Schwartz PJ, Crotti L, Insolia R.. Long-QT syndrome from genetics to management. Circ Arrhythm Electrophysiol 2012; 5: 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Payne RB, Little AJ, Williams RB, Milner JR.. Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 1973; 4: 643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al.. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 17. Aonuma K.. Guidelines for diagnosis and management of inherited arrhythmias (JCS 2017). http://www.j-circ.or.jp/guideline/pdf/JCS2017_aonuma_h.pdf (accessed February 8, 2021).
- 18. Itoh H, Crotti L, Aiba T, Spazzolini C, Denjoy I, Fressart V, et al.. The genetics underlying acquired long QT syndrome: Impact for genetic screening. Eur Heart J 2016; 37: 1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim IJ, Yang PS, Kim TH, Uhm JS, Pak HN, Lee MH, et al.. Relationship between anemia and the risk of sudden cardiac arrest: A nationwide cohort study in South Korea. Circ J 2018; 82: 2962–2969. [DOI] [PubMed] [Google Scholar]
- 20. Liu P, Wang L, Han D, Sun C, Xue X, Li G.. Acquired long QT syndrome in chronic kidney disease patients. Ren Fail 2020; 42: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherif KA, Abo-Salem E, Panikkath R, Nusrat M, Tuncel M.. Cardiac repolarization abnormalities among patients with various stages of chronic kidney disease. Clin Cardiol 2014; 37: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raizada V, Hillerson D, Amaram JS, Skipper B.. Angiotensin II-mediated left ventricular abnormalities in chronic kidney disease. J Investig Med 2012; 60: 785–791. [DOI] [PubMed] [Google Scholar]
- 23. Raizada V, Skipper B, Luo W, Garza L, Hines CW, Harford AA, et al.. Renin-angiotensin polymorphisms and QTc interval prolongation in end-stage renal disease. Kidney Int 2005; 68: 1186–1189. [DOI] [PubMed] [Google Scholar]
- 24. Napolitano C.. Evidence for a cardiac ion channel mutation underlying drug-induced QT prolongation and life-threatening arrhythmias. J Cardiovasc Electrophysiol 2000; 11: 691–696. [DOI] [PubMed] [Google Scholar]
- 25. Shimaoka T, Wang Y, Morishima M, Miyamoto S, Ono K.. Magnesium deficiency causes transcriptional downregulation of Kir2.1 and Kv4.2 channels in cardiomyocytes resulting in QT interval prolongation. Circ J 2020; 84: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 26. Makita N, Horie M, Nakamura T, Ai T, Sasaki K, Yokoi H, et al.. Drug-induced long-QT syndrome associated with a subclinical SCN5A mutation. Circulation 2002; 106: 1269–1274. [DOI] [PubMed] [Google Scholar]
- 27. Itoh H, Sakaguchi T.. Latent genetic backgrounds and molecular pathogenesis in drug-induced long-QT syndrome. Circ Arrhythm Electrophysiol 2009; 2: 511–523. [DOI] [PubMed] [Google Scholar]
- 28. De Ponti F, Poluzzi E, Montanaro N.. QT-interval prolongation by non-cardiac drugs: Lessons to be learned from recent experience. Eur J Clin Pharmacol 2000; 56: 1–18. [DOI] [PubMed] [Google Scholar]
- 29. Drew BJ.. Prevention of torsade de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010; 121: 1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The Japan Geriatrics Society.. Chapter 4-2, Neurological disorder. In: The Japan Geriatrics Society Working Group, editors. Guidelines for medical treatment and its safety in the elderly 2015. Tokyo: Medical View, 2015; 52–59 [in Japanese]. [Google Scholar]