Figure 1.
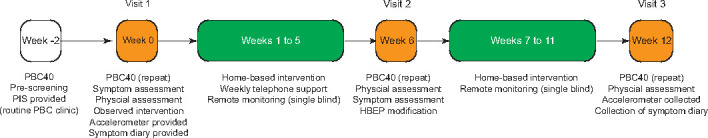
Study overview. Patients with moderate-severe fatigue (PBC40 fatigue domain score >33) will be identified from clinic and PBC40 fatigue assessment completed. Eligible participants will be invited to attend a dedicated screening visit within 2 weeks and a repeat PBC40 questionnaire completed. After obtaining consent, the investigator will perform full physical and symptom assessment of the trial participant and demonstrate the intended intervention. The trial participant will then be observed while performing the aforementioned intervention and will be provided a symptom diary and single-blinded GeneActiv accelerometer for home use (daily activity monitor readings will be captured remotely by the investigator, but not visible by the participant). Thereafter, the participant will be instructed to perform a tailored, daily HBEP by the investigators (liver physiotherapist and personal trainer). Weekly telephone support will be provided in the first 6 weeks (interval between visit 1 and visit 2), together with modifications to the exercise programme as needed. At week 6, the trial participant will be invited for an interim assessment, followed by another 6 weeks of intervention. Weekly telephone support will be withdrawn between weeks 6 and 12 (end of the study). Assessment of the primary efficacy measure will be performed at week 12 (end of study visit). HBEP, home-based exercise programme; PBC, primary biliary cholangitis; PIS, patient information sheet.
