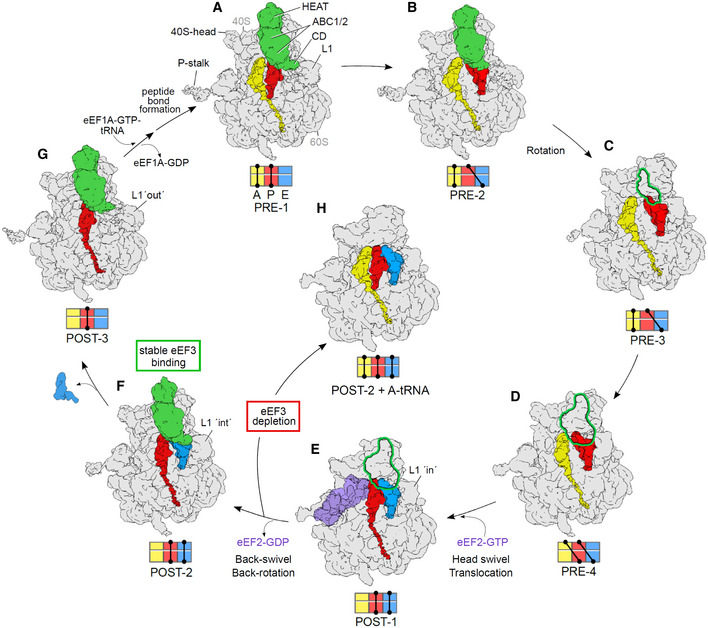
Figure 7. Functional role of eEF3 in the framework of the elongation cycle.
-
A, BeEF3 binds to (A) non‐rotated 80S in a pre‐translocation (PRE) state bearing classical A/A‐ and P/P‐tRNA (PRE‐1) as well as to (B) non‐rotated state occupied by A/A‐ and a hybrid P/E‐tRNA (PRE‐2).
-
C, D(C) Rotation of the ribosomal subunits leads to unstable binding of eEF3 to a ribosome with A/A‐ and P/E‐tRNA (PRE‐3) as well as to (D) a fully rotated ribosomal species bearing hybrid A/P‐ and P/E‐tRNAs (PRE‐4).
-
EBinding of eEF2‐GTP facilitates 40S‐head swiveling and translocation of the hybrid A/P‐ and P/E‐tRNA into the chimeric ap/P and pe/E‐tRNA positions (POST‐1).
-
FAfter dissociation of eEF2‐GDP and the resulting back‐swivel and back‐rotation of the ribosome, eEF3 binds stably to the non‐rotated ribosome with a classical P/P‐ and E/E‐tRNA (POST‐2) with L1‐stalk in the “int” position.
-
GThe eEF3‐CD directly interacts with L1 stabilizing an “out” conformation that facilitates release of the E‐site tRNA. eEF3 remains bound to the non‐rotated ribosome bearing a P/P‐tRNA (POST‐3). Transition from states E to F and F to G are accelerated by eEF3. Binding of eEF1a‐ATP‐tRNA ternary complex and subsequent peptide bond formation results in formation of PRE‐1, as seen in (A).
-
HIn the absence of eEF3, we suggest that POST‐2 ribosomes formed after eEF2 dissociation can bind an A‐tRNA, but cannot translocate further because of the presence of three tRNAs on the ribosome.
Data information: Volumes (A–G) are representing the cryo‐EM reconstructions from the eEF3‐TAP pull‐out, whereas volume (H) shows a potential scenario based of the results of the eEF3‐depletion studies. All maps are filtered to 6 Å. The outline shown in (C–E) assigns the disordered eEF3 ligand present in these volumes.
