Figure 7. G187 is critical for determining the sensitivity of the GRAM domain to accessible PM cholesterol and regulating GRAMD1b‐dependent cholesterol transport.
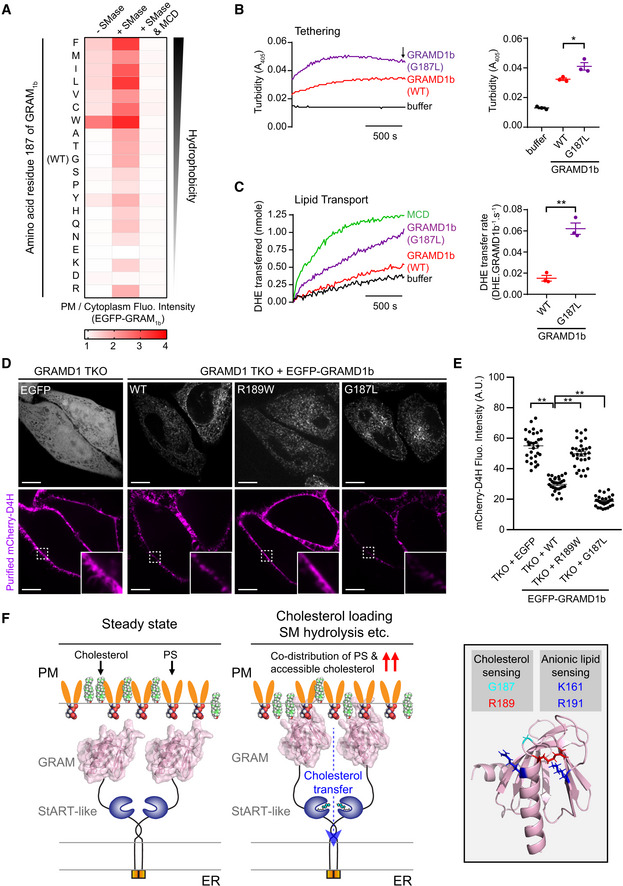
- Effects of mutation of G187 on the property of the GRAM domain of GRAMD1b (GRAM1b) to sense transient expansion of the accessible pool of PM cholesterol. Indicated EGFP‐tagged mutant versions of GRAM1b were expressed in GRAMD1 TKO HeLa cells and imaged under confocal microscopy with or without SMase treatment (100 mU/ml for 1 h at 37°C) or with SMase treatment followed by 5 min treatment with Methyl‐β‐cyclodextrin (MCD) (5 mM) to assess their PM recruitment. The mean values (n = 20 cells from two independent experiments for untreated and SMase‐treated conditions; n = 10 cells from one experiment for SMase & MCD‐treated conditions) of the ratio of PM signals to the cytosolic signals, as assessed by line scan analysis, are presented for each condition as a heatmap. Amino acids are ranked according to Goldman, Engelman and Steitz (GES) hydrophobicity scale with the most hydrophobic amino acid on the top. Individual values for each condition are shown in Fig EV5A.
- Liposome tethering by purified GRAMD1b proteins. Left: Representative time course of liposome tethering, as assessed by turbidity of mixtures containing both LPM and LER together with either wild‐type GRAMD1b proteins (WT), mutant GRAMD1b G187L proteins (G187L), or buffer alone as indicated. Experiments and data analysis were performed as in Fig 4. Right: Values of turbidity corresponding to the end of the experiment as indicated by the arrow (mean ± SEM, n = 3 independent experiments; Dunnett’s multiple comparisons test, *P = 0.0143).
- DHE transfer between liposomes by purified GRAMD1b proteins. Left: Representative time course of DHE transfer from LPM to LER mediated by either wild‐type GRAMD1b proteins (WT), mutant GRAMD1b G187L proteins (G187L), or buffer alone as indicated. Methyl‐β‐cyclodextrin (MCD) at 1 mM was used to determine DHE equilibration. Experiments and data analysis were performed as in Fig 4. Right: Values of DHE transfer rate of wild‐type GRAMD1b (WT) and mutant GRAMD1b (G187L), as estimated by the FRET‐based lipid transfer assay (mean ± SEM, n = 3 independent experiments; two‐tailed unpaired Student’s t‐test, **P < 0.0014).
- Confocal images of live GRAMD1 TKO HeLa cells that stably expressed either EGFP control or EGFP‐tagged GRAMD1b (EGFP‐GRAMD1b) constructs as indicated. Cells were stained with recombinant mCherry–D4H proteins (10 mg/ml) (accessible PM cholesterol biosensor) for 15 min at room temperature before imaging. Insets show at higher magnification the regions indicated by white dashed boxes. Note that expression of EGFP‐GRAMD1b–WT, but not EGFP‐GRAMD1b–R189W, reduced binding of mCherry‐D4H to the PM, in GRAMD1 TKO HeLa cells. Further reduction in mCherry‐D4H binding to the PM was observed in GRAMD1 TKO HeLa cells that stably expressed EGFP‐GRAMD1b–G187L compared to GRAMD1 TKO HeLa cells that stably expressed EGFP‐GRAMD1b–WT. Scale bars, 10 µm.
- Values of mCherry‐D4H signals at the PM after background subtraction, as assessed by confocal microscopy and line scan analysis of GRAMD1 TKO HeLa cells that stably expressed either EGFP or indicated EGFP‐GRAMD1b constructs as shown in (D) [mean ± SEM, n = 30 cells for all the conditions; data are pooled from three independent experiments; Dunnett’s multiple comparisons test, **P < 0.0001].
- A model of the molecular basis of accessible PM cholesterol recognition by the GRAM domain of GRAMD1b. At steady state, limited amount of accessible cholesterol (i.e. chemically active cholesterol) in the inner leaflet of the PM is not sufficient for the recruitment of the GRAM domain to the PM. Upon additional cholesterol loading to the PM or liberation of cholesterol from sphingomyelin (SM)‐sequestered pool of inaccessible PM cholesterol (i.e. chemically inactive cholesterol), accessible PM cholesterol transiently increases in the inner leaflet of the PM beyond a certain threshold (as unsequestered cholesterol can spontaneously flip‐flop between the outer and inner leaflets), resulting in increased codistribution of accessible cholesterol and anionic lipids, including phosphatidylserine (PS), in this leaflet, which serves as a platform for PM recruitment of the GRAM domain. The GRAM domain then binds to both accessible cholesterol and anionic lipids, using distinct but synergistic binding sites for cholesterol and anionic lipids, to tether the PM to the ER, and facilitates the StART‐like domain‐dependent extraction and transport of accessible PM cholesterol to the ER. Accessible cholesterol transport to the ER results in suppression of SREBP‐2 cleavage, preventing overaccumulation of cholesterol in cells. Our study identified amino acid residues that are important for cholesterol sensing (G187, R189) and anionic lipid sensing (K161, R191) of the GRAM domain. G187 is critical for determining the sensitivity of the GRAM domain to accessible PM cholesterol, while K161 and R191 contribute to a basic patch of the GRAM domain that is required for sensing anionic lipids, including PS. An intellectual disability‐associated R189W mutation, which specifically results in cholesterol‐sensing defect, impairs the GRAM domain’s ability to sense transient expansions of the accessible pool of PM cholesterol and abolishes GRAMD1b‐mediated PM to ER cholesterol transport.
