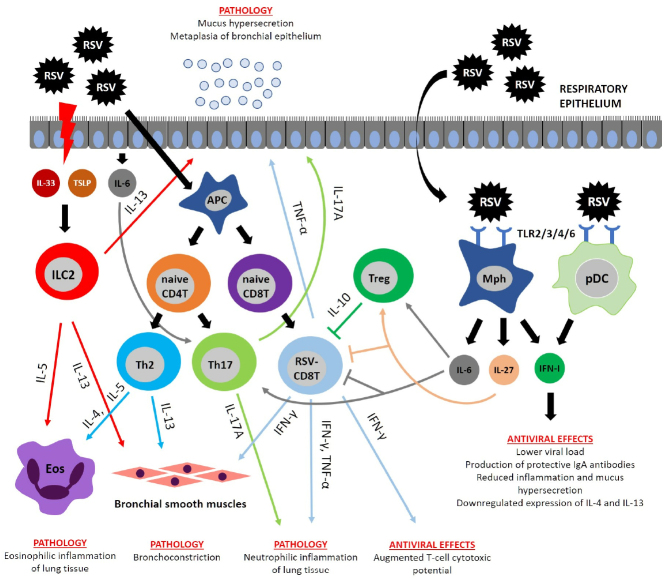
Fig. 2.
Immunopathogenesis of RSV infection. RSV infection activates proliferation of CD8 T cells producing IFNγ and TNFα involved in the emergence of major pathology manifestations. IFNγ not only participates in the pathology development, but also accounts for the host antiviral defense. RSV induces necrosis in the respiratory epithelium, resulting in the release of cytokines IL-33 and thymic stromal lymphopoietin (TSLP) that activate ILC2 cells producing their own cytokines (IL-5 and IL-13). IL-5 and IL-13 are involved in the development of pathological manifestations by causing pulmonary eosinophilic inflammation, mucus hypersecretion, and BHR. Polarization of Th2 and Th17 cells occurs during RSV infection due to specific cytokine microenvironment. Th2 cells produce cytokines IL-4, IL-5, and IL-13, which trigger BHR, mucus hypersecretion, and pulmonary eosinophilic inflammation, whereas Th17 cells secrete IL-17A that induces pulmonary neutrophilic inflammation and mucus hypersecretion. Immunoregulatory functions are executed by the cytokine IL-10 released by regulatory T cells (Treg). IL-10 suppresses the pro-inflammatory activity of CD8 T cells. Similar activity was described for IL-6 and IL-27, which are able to directly suppress CD8 T cells and activate Treg. RSV is recognized by surface TLR proteins on the macrophages (MPhs) and plasmacytoid DCs (pDCs) producing limited amounts of type I interferons (IFN-I) with the antiviral activity.