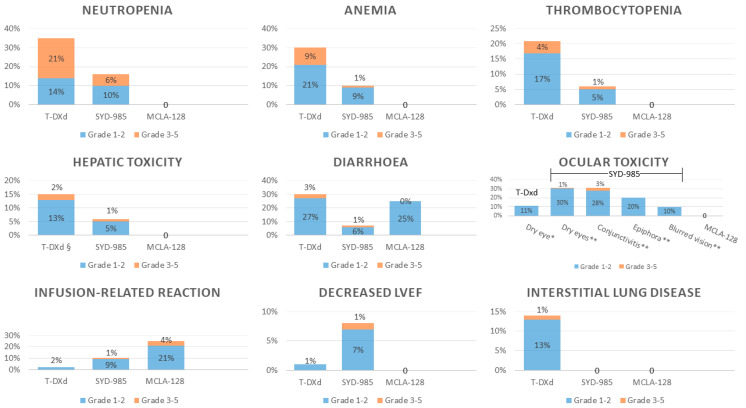
Figure 2.
Incidence of key adverse events induced by trastuzumab–deruxtecan, trastuzumab–duocarmazine and zenocutuzumab. Abbreviations: T-DXd: trastuzumab–deruxtecan, SYD-985: trastuzumab–duocarmazine, MCLA-128: zenocutuzumab, LVEF: left ventricular ejection fraction. § Data extracted from all enrolled patients (dose-escalation and dose-expansion parts). * The only reported ocular adverse event was dry eye (data extracted from all enrolled patients (dose-escalation and dose-expansion parts)). ** other non-reported ocular adverse events include episcleritis (3%), corneal toxicity (1%) and retinal hemorrhage (1%).