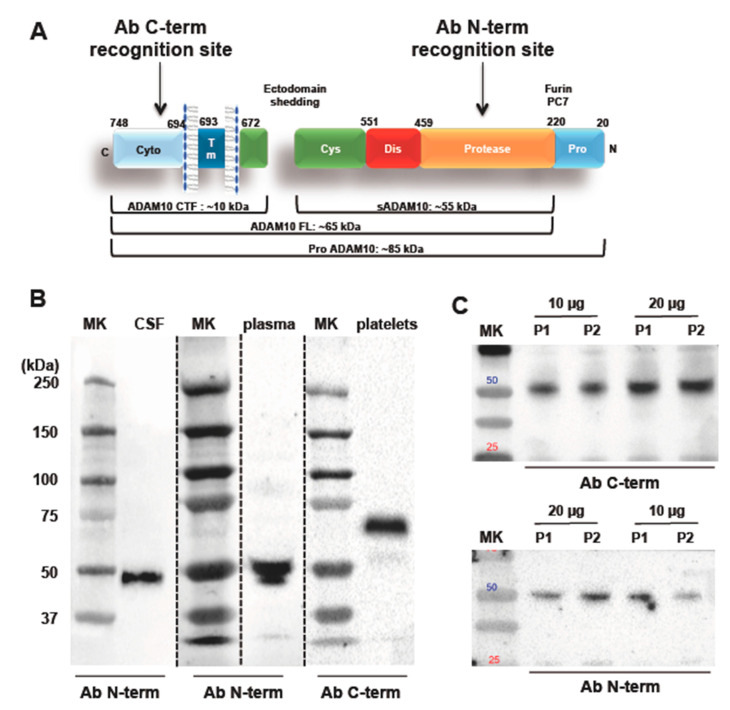
Figure 1.
The ADAM10 multimodular structure and its different forms. (A) Furin/PC7 proteases cleave Pro-ADAM10, the inactive protein containing a pro-domain (85 kDa), to activate the enzyme during the transit through the Golgi compartment. The full-length active form (ADAM10 FL, 60–65 kDa) is then directed to the cell membrane where it works as sheddase. ADAM10 itself can be subject to ectodomain shedding, a process that leads to the formation of the soluble (sADAM10, 50–55 kDa) and membrane-anchored C-terminal domain (ADAM10 CTF, 10 kDa). Antibody binding sites used in this study to recognize ADAM10 protein. (B) Western blotting membranes using the ProSci 2051 antibodies (detection of the C-terminal region) in platelets and Abcam 39153 (detection of the N-terminal region) in plasma and CSF. MK: standard marker. (C) Western Blot analysis of 10 μg and 20 μg aliquots of plasma obtained from two control subjects (P1 and P2). Both ADAM10 antibodies recognize a band with an apparent molecular weight of 50 kDa.