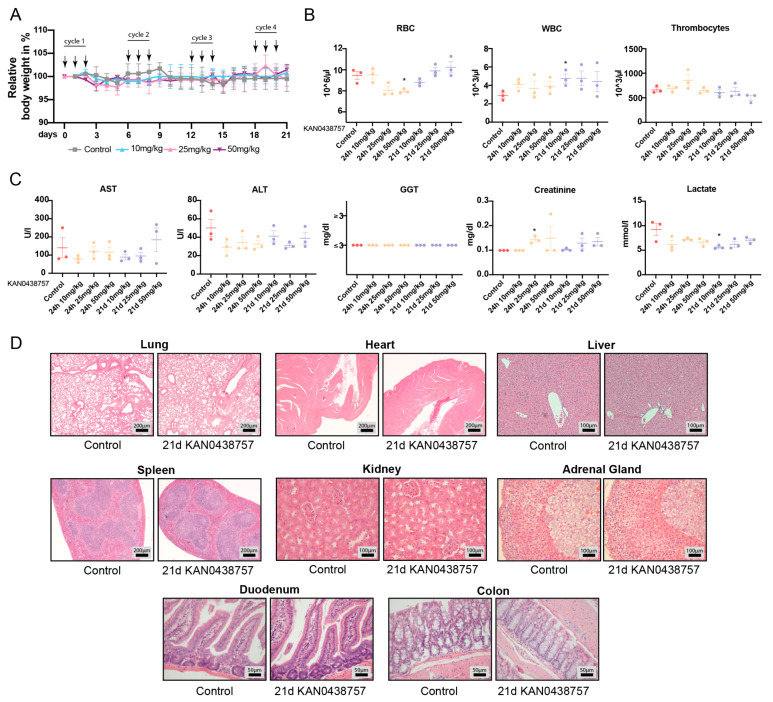
Figure 7.
In vivo toxicity testing of KAN0438757 in immune-competent mice. (A) Relative weight curves of C57BL6/N mice treatment with 10 mg/kg (n = 3), 25mg/kg (n = 3) and 50 mg/kg (n = 3) KAN0438757 (arrows, i.p. injections). Control, DMSO (n = 3). (B,C) Whole blood counts (RBC, WBC, thrombocytes) and serum biochemical evaluation of aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT), creatinine and lactate after treatment periods of 24 h to 21 days with KAN0438757. (D) Representative pictures of histological analysis (H&E) of lungs, heart, liver, spleen, kidneys, adrenal glands, duodenum and colon from mice treated 21 days (21 d) with KAN0438757 (25 mg/kg dosage). * p < 0.05; Data is SEM.