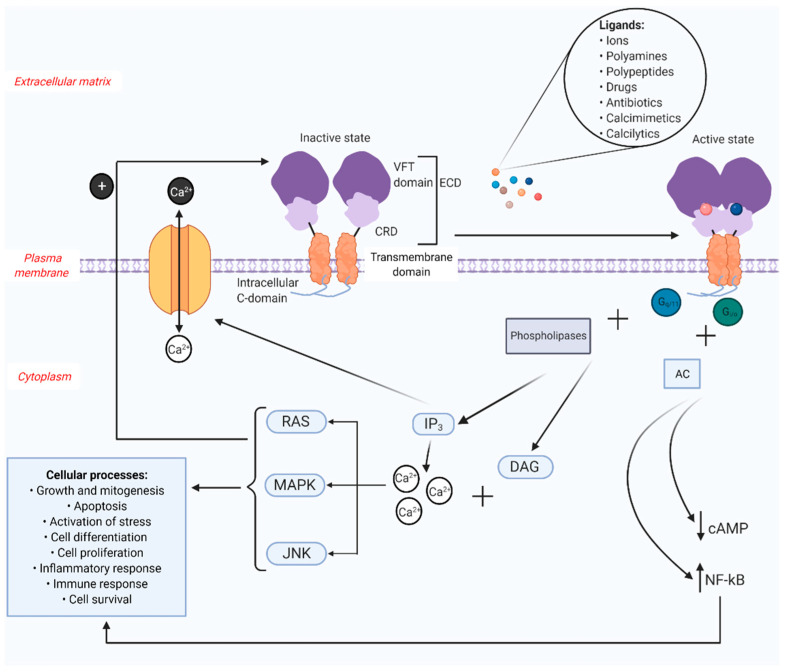
Figure 1.
Structure and function of CaSR. The CaSR is composed of three structural domains: the extracellular domain (ECD) consisting of a Venus flytrap (VFT) domain and a cysteine rich domain (CRD); the transmembrane domain connected by the CRD to the VFT; and an intracellular C-domain. The extracellular ligands of CaSR include various ions, polyamines, polypeptides, drugs, antibiotics, calcimimetics and calcilytics. These ligands bind to the inactive receptor to initiate conformational changes to the receptor resulting in receptor activation. The active receptor differs from the inactive receptor in such a way that the protomers in the VFT are in close proximity to each other. The surface receptor, when activated by its ligands, can trigger numerous intracellular signaling pathways, which in turn activate different cellular processes. The activation of CaSR couples Gi/o and Gq/11 families of heterotrimeric G proteins. Along with phospholipases, Gq/11 prompts inositol triphosphate (IP3) and diacylglycerol (DAG) pathways. IP3 controls calcium channels on the surface of the cell, thereby regulating the efflux and influx of calcium ions. DAG, along with the cytosolic calcium will activate rat sarcoma (RAS)/mitogen-activated protein kinase (MAPK)/c-Jun n-terminal kinases (JNK) pathways which are involved in the initiation of several cellular processes. These pathways also engage in a feedback mechanism required for the proper functioning of the receptor. Gi/o interacts with adenylate cyclase (AC) to curb the production of cyclic adenosine monophosphate (cAMP). The receptor also upregulates the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), which is again associated with various cellular processes (Created with BioRender.com).