Abstract
Background
Programmed death ligand 1 (PD‐L1) has been used as a diagnostic marker to identify patients that will benefit from immune checkpoint inhibitors in non‐small cell lung cancer (NSCLC). Immunohistochemistry with E1L3N clone is one of the most widely used and inexpensive laboratory‐developed tests for PD‐L1, but still need to be compared and validated with standard methods for clinical application.
Methods
We investigated the performance of E1L3N clone for PD‐L1 testing in 299 tumor tissues of NSCLC patients and its comparability with FDA‐approved 22C3 clone.
Results
The results show that the negative coincidence rate, weak positive coincidence rate, and positive coincidence rate were 97.4%, 92.2%, and 97.6% using the E1L3N assay relative to the 22C3 assay, respectively. An overall agreement of 96.3% was achieved between these two assays. We also found that the overall concordances were 97.8% and 93.9% for PD‐L1 detection in large and small specimens, respectively, and no significant difference was obtained between these two assays (p = 0.076). In addition, the expression of PD‐L1 was not detected in tumor tissues of benign lung disease using both the E1L3N and 22C3 assays.
Conclusion
E1L3N can be used as a reliable alternative antibody clone to evaluate PD‐L1 expression status for NSCLC patients.
Keywords: consistency evaluation, E1L3N, immunotherapy, non‐small cell lung cancer, PD‐L1
Programmed death ligand 1 (PD‐L1) has been used as a diagnostic marker to select patients that will benefit from immune checkpoint inhibitors in non‐small cell lung cancer (NSCLC). Here, we investigated the performance of E1L3N clone, one of the most widely used and inexpensive laboratory‐developed tests, for PD‐L1 testing in 299 tumor tissues of NSCLC patients and its comparability with FDA‐approved 22C3 clone. Our results reveal that E1L3N can be used as a reliable alternative antibody clone to evaluate PD‐L1 expression status for NSCLC patients.
1. INTRODUCTION
Lung cancer is one of the leading causes of cancer‐related death in the world. 1 Non‐small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers, of which adenocarcinoma and squamous cell carcinoma are the most common subtypes. 2 In recent years, the treatment protocols of NSCLC patients have been dramatically developed, especially the usage of tyrosine kinase inhibitors in patients with EGFR, ALK, and ROS1 mutations. 2 However, inhibitors of programmed death 1 (PD‐1) and its ligand PD‐L1 could be an effective therapeutic strategy to improve the survival rate of NSCLC patients without gene mutations. 3 , 4
Despite the emergence of new drugs in immunotherapy, it is still important to evaluate the beneficiaries accurately for the treatment of NSCLC patients. 5 Currently, PD‐L1 is the only diagnostic marker approved in clinical practice for immunotherapy, and immunohistochemistry (IHC) has been widely applied in PD‐L1 detection due to its high efficiency and fast analysis. 6 So far, four standardized PD‐L1 IHC assays have been approved by the Food and Drug Administration (FDA) for clinical application, including 22C3 and 28‐8 pharmDx on the Dako platform, SP142 and SP263 on the Ventana platform as well as PD‐L1 IHC 22C3 pharmDx. 7 , 8 However, the predictive and prognostic performances of PD‐L1 vary considerably due to differences in antibody clones, IHC platforms, detection systems, and scoring algorithms. 9 Munari et al. 10 reported that the 22C3 and SP263 assays showed variable results for identifying PD‐L1 positive cases, resulting in underestimation of patients for pembrolizumab therapy. In addition, of note, the Dako and Ventana platforms are not universally available and the standardized PD‐L1 assays are expensive for patients with financial difficulties. Excepting NSCLC, PD‐1/PD‐L1 inhibitors have also been developed for the treatment of other cancers, such as primary adrenal lymphoma, 11 gastric cancer, 12 kidney cancer, 13 and liver cancer. 14 Thus, developing inexpensive and universal laboratory‐developed tests has been strongly advocated by pathologists to make PD‐L1 testing broadly available. 15
The clone E1L3N from the Cell Signaling Technology is one of the most commonly used and inexpensive PD‐L1 antibodies. However, its diagnostic results were always conflicting when compared with standardized assays. 8 , 16 , 17 For example, Cogswell et al. 18 reported that E1L3N was more sensitive than 28‐8 using the identical detection method, whereas a contrary result was obtained using the manufacturers’ method. Munari et al. 19 found high concordance for the evaluation of PD‐L1 expression between E1L3N and SP263, but low concordance between E1L3N and 22C3. However, another study enrolled 100 NSCLC patients showed that the SP263 assay was more sensitive for detecting PD‐L1 expression in tumor cells and immune cells than the E1L3N assay. 20 Therefore, the clinical application of PD‐L1 E1L3N assay still need to be further analyzed and validated. In this study, we evaluate the performance of the E1L3N assay for detecting PD‐L1 expression in NSCLC tumor tissues compared with the 22C3 assay. Moreover, the consistency of E1L3N clone in large and small tissue specimens of NSCLC patients was also assessed for the detection of PD‐L1 expression.
2. MATERIALS AND METHODS
2.1. Patients
This prospective study was carried out in the Department of Pulmonary and Critical Care Medicine at the First Affiliated Hospital of Wenzhou Medical University (Zhejiang, China). Patients were enrolled according to the following inclusion criteria from May 1, 2019, to October 25, 2019: (1) age ≥18 years; (2) patients were pathologically confirmed NSCLC or benign lung disease according to the Eighth Edition of TNM stage classification of malignant tumors 21 ; (3) tissue collection was no more than one month or the samples can be obtained and tested within 1 month; (4) enough formalin‐fixed, paraffin‐embedded (FFPE) samples (six slices of 3–5 μm thickness); (5) patients voluntarily joined the study and signed the informed consent. Exclusion criteria included the following: (1) the tissue samples contained very few tumor cells (total number of tumor cells <100); (2) FFPE samples were not kept as required; (3) patients were accompanied with other malignant disease or autoimmune diseases. The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University (No. 2019032). All patients provided written informed consent before the collection and use of their tissues.
2.2. Specimen collection and preparation
In this study, specimens were collected from each patient and kept as FFPE tissue samples. Six sections (3–5 μm thickness) of each qualified tissue samples were used for PD‐L1 detection. All samples were tested for PD‐L1 expression by PD‐L1 IHC E1L3N assay (Amoy Diagnostics) and verified by FDA‐approved diagnostic assay PD‐L1 IHC 22C3 pharmDx (Agilent Technologies/Dako). For the samples with inconsistent results, a third reagent PD‐L1 IHC SP263 assay (Roche Ventana) was employed for further verification.
Two consecutive sections were prepared according to the product specifications of the PD‐L1 IHC E1L3N assay, PD‐L1 IHC 22C3 pharmDx, and PD‐L1 IHC SP263 assay. The specific requirements included as follows: (1) E1L3N: the section was placed on an adhered glass slide and baked at 65°C for 2 h. If the section cannot be tested immediately, it should be stored at 2–8°C and detected within 6 months; (2) 22C3: the section was placed on an adhered glass slide and baked at 58°C for 1 h. If the section cannot be tested immediately, it should be stored at 2–8°C and detected within 1 month; (3) SP263: the section was placed on the adhered slide and detected within 6 months.
2.3. The detection of PD‐L1 expression using different antibody clones
In this study, two consecutive sections were simultaneously used for evaluating different antibody reagents (E1L3N, 22C3 and SP263) and their corresponding control reagents according to the manufacturer's instructions. PD‐L1 expressions were detected by PD‐L1 IHC E1L3N assay kit according to the manufacturer's instructions. Two prepared serial sections were applied for the simultaneous detection experiment of the PD‐L1 IHC E1L3N assay reagent and the blank control reagent. The E1L3N assay was carried out with the Leica BOND‐MAX automatic immunohistochemical strainer. The 22C3 assay was performed using the DakoAutostainer Link 48 automatic immunohistochemical staining system. In addition, the SP263 assay was conducted by the Ventana Benchmark Ultra automatic immunohistochemical staining system.
The Tumor Proportion Score (TPS) was calculated as the percentage of viable PD‐L1‐positive tumor cells in all viable tumor cells in the section. The PD‐L1 negative, weak positive, and positive samples were defined when TPS < 1%, 1% ≤ TPS < 50%, and TPS ≥ 50%, respectively. Representative images of PD‐L1 expression detected by the E1L3N and 22C3 assays were illustrated in Figure 1.
FIGURE 1.
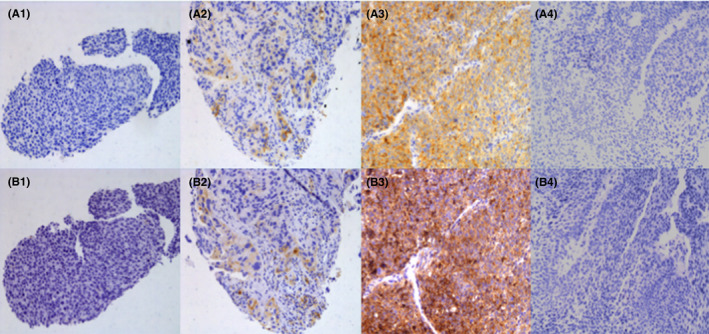
Representative immunohistochemistry images of PD‐L1 expression using the clone E1L3N (A1–A4) and 22C3 (B1–B4). (A1, B1) Tumor proportion score (TPS) <1%; (A2, B2) 1% ≤ TPS <50%; (A3, B3) TPS ≥ 50%; (A4, B4) Tumor tissues of benign lung disease. TPS was calculated as the percentage of viable PD‐L1‐positive tumor cells in all viable tumor cells in the section. Magnification: 200×
2.4. Statistical analysis
The expression of PD‐L1 in tissue samples detected by the 22C3 assay was considered to be the standard for the consistency evaluation of the E1L3N assay. The degree of agreement was analyzed by the Kappa test. The consistency between the E1L3N and 22C3 assays for detecting PD‐L1 expression in large and small tissue specimens was measured by the two‐sided chi‐square test using IBM SPSS Statistics ver. 23.0 (IBM Corp.). Differences between two groups were considered to be statistically significant when p < 0.05.
3. RESULTS
3.1. Patient characteristics
In this study, a total of 343 patients met the enrollment criteria and were prospectively entered into the study. Thirty‐one patients were excluded, of which 25 samples had very few tumor cells after histopathologic examination and six samples had insufficient tissue samples. Finally, 312 patients were used for further analysis, including 299 patients with NSCLC and 13 patients with benign lung disease. Of the 13 patients with benign lung disease, there were 3 (23.1%) patients with lung hamartoma, 2 (15.4%) patients with tuberculosis, 4 (30.8%) patients with granulomatous inflammation, and the others with chronic inflammatory disease. The number of males and females was 4 (30.8%) and 9 (69.2%), respectively. Of the 299 patients initially diagnosed with NSCLC, 223 (74.6%) patients were confirmed as adenocarcinoma and 70 (23.4%) patients as squamous cell lung cancer by histopathology. In addition, 286 (95.7%) patients were newly diagnosed in this study. Among these FFPE tissue samples, 184 (61.5%) samples were collected via surgery operation, 65 (21.7%) samples via pulmonary aspiration, and 43 (14.4%) samples via bronchoscopy. The detailed clinical characteristics of lung cancer patients are listed in Table 1.
TABLE 1.
Clinical characteristics of patients with non‐small cell lung cancer
| Characteristics | n | Proportion (%) |
|---|---|---|
| Age (years) | ||
| <60 | 105 | 35.1 |
| ≥60 | 194 | 64.9 |
| Sex | ||
| Male | 179 | 59.9 |
| Female | 120 | 40.1 |
| Pathological type | ||
| Adenocarcinoma | 223 | 74.6 |
| Squamous cell | 70 | 23.4 |
| Large cell | 4 | 1.3 |
| Others | 2 | 0.7 |
| Stage | ||
| I | 151 | 50.5 |
| II | 20 | 6.7 |
| III | 45 | 15.0 |
| IV | 83 | 27.8 |
| Stage of treatment | ||
| Newly diagnosed | 286 | 95.7 |
| Progression | 10 | 3.3 |
| Recurrence after surgery | 3 | 1.0 |
| EGFR/ALK/ROS1gene | ||
| Positive | 47 | 15.7 |
| Negative | 48 | 16.1 |
| No detection | 204 | 68.2 |
| Tissue collection | ||
| Bronchoscope | 43 | 14.4 |
| Pulmonary aspiration | 65 | 21.7 |
| Surgical operation | 184 | 61.5 |
| Metastatic sites | ||
| Lymph nodes | 4 | 1.4 |
| Pleura | 2 | 0.7 |
| Bone | 1 | 0.3 |
3.2. Comparison of PD‐L1 testing in tumor tissues using the E1L3N and 22C3 assays
Among all 299 NSCLC patients, PD‐L1 expression was detected in 106 (35.5%) samples using the E1L3N assay, of which 41 (38.7%) and 65(61.3%) samples were defined as positive and weak positive, respectively, as shown in Table 2. In addition, the E1L3N assay detected 193 (64.5%) samples as PD‐L1 negative (Table 2). Using the 22C3 assay, 193 (64.5%) samples were determined as PD‐L1 negative, 64 (21.4%) samples as weak positive, and 42 (14.1%) samples as positive (Table 2). We also found that PD‐L1 expression was not detected in 13 samples of patients with benign lung diseases using both the E1L3N and 22C3 assays. Compared with the 22C3 assay, the negative coincidence rate (NCR), weak positive coincidence rate (WPCR), and positive coincidence rate (PCR) were 97.4% (94.1–99.2%), 92.2% (82.7–97.4%), and 97.6% (87.4–99.9%), respectively. The negative predictive value (NPV), weak positive predictive value (WPPV), and positive predictive value (PPV) were 97.4% (94.1–99.2%), 90.8% (81.0–96.5%), and 100.0% (91.4–100.0%), respectively. Of the 299 tumor tissue samples, 288 (96.3%) samples presented the consistent PD‐L1 expression status using both the E1L3N and 22C3 assays. The degree of concordance for testing PD‐L1 expression in tumor tissues between these two assays was evaluated by the Kappa test, and a coefficient of 0.929 (p < 0.001) was achieved in this study.
TABLE 2.
Comparison of the detection of PD‐L1 expression in tumor tissues between the E1L3N and 22C3 assays
| 22C3 | |||||
|---|---|---|---|---|---|
| Neg a | WPos b | Pos c | Total | ||
| E1L3N | Neg | 188 | 5 | 0 | 193 |
| WPos | 5 | 59 | 1 | 65 | |
| Pos | 0 | 0 | 41 | 41 | |
| Total | 193 | 64 | 42 | 299 | |
| NPV d | 97.4% (94.1%−99.2%) j | ||||
| WPPV e | 90.8% (81.0%−96.5%) | ||||
| PPV f | 100.0% (91.4%−100.0%) | ||||
| NCR g | 97.4% (94.1%−99.2%) | ||||
| WPCR h | 92.2% (82.7%−97.4%) | ||||
| PCR i | 97.6% (87.4%−99.9%) | ||||
| Overall agreement | 96.3% (93.5%−98.2%) | ||||
Negative;
Weak positive;
Positive;
Negative Predictive Value;
Weak positive predictive value;
Positive Predictive Value;
Negative coincidence rate;
Weak positive coincidence rate;
Positive coincidence rate;
95% confidence interval.
There were 11 cases with inconsistent results of PD‐L1 expression detected by the E1L3N and 22C3 assays, as shown in Table 3. In these 11 cases, five patients who detected as weak positive by the 22C3 assay were identified as PD‐L1 negative using the E1L3N assay. On the contrary, another five samples were detected as weak positive by the E1L3N assay but negative by the 22C3 assay. In addition, one patient with PD‐L1 positive based on the 22C3 assay was identified as weak positive by the E1L3N assay. Subsequently, the expressions of PD‐L1 in these 11 cases were validated by the SP263 assay. The results show that there were four cases in the E1L3N assay and seven cases in the 22C3 assay that were in agreement with the SP263 assay (Table 3).
TABLE 3.
Validation of patients with inconsistent PD‐L1 expression using the SP263 assay
| No | E1L3N | 22C3 | SP263 |
|---|---|---|---|
| 1 | Weak positive | Negative | Weak positive |
| 2 | Weak positive | Negative | Negative |
| 3 | Negative | Weak positive | Weak positive |
| 4 | Negative | Weak positive | Weak positive |
| 5 | Weak positive | Positive | Positive |
| 6 | Weak positive | Negative | Weak positive |
| 7 | Negative | Weak positive | Weak positive |
| 8 | Negative | Weak positive | Weak positive |
| 9 | Negative | Weak positive | Weak positive |
| 10 | Weak positive | Negative | Weak positive |
| 11 | Weak positive | Negative | Weak positive |
3.3. Evaluation of PD‐L1 expression in large and small tumor tissues using the E1L3N and 22C3 assays
Of the 299 FFPE tissue samples, we collected 184 (61.5%) samples via surgery operation, 65 (21.7%) samples via pulmonary aspiration, 43 (14.4%) samples via bronchoscopy, 4 (1.3%) samples via lymph node biopsy, 2 (0.7%) samples via pleura biopsy, and 1 (0.4%) sample via bone biopsy in operating room. Surgical and bone biopsy specimens are defined as large tissue samples, while specimens collected by pulmonary aspiration, bronchoscopy, lymph node biopsy, and pleura biopsy are defined as small tissue samples.
Of the 185 large specimens, there were 125 negative, 40 weak positive, and 20 positive PD‐L1 expressions detected by the E1L3N assay, while 127 negative, 38 weak positive, and 20 positive PD‐L1 expressions were obtained using the 22C3 assay (Table 4). Only four specimens had an inconsistent result. The overall concordance between these two assays in large tissue specimens was 97.8% (95% CI, 94.6–99.4%). Of the 114 small specimens, we identified 68 samples as PD‐L1 negative, 25 samples as weak positive, and 21 samples as positive using the E1L3N assay. Using the 22C3 assay, however, there were 66, 26, and 22 samples detected as PD‐L1 negative, weak positive, and positive, respectively. A total of seven small specimens had inconsistent results, and the overall concordance between these two assays in small tissue specimens was 93.9% (95% CI, 87.8–97.5%). Moreover, there was no significant difference in the detection of PD‐L1 expression in large and small tissue specimens between the E1L3N and 22C3 assays (p = 0.076).
TABLE 4.
Comparison of the detection of PD‐L1 expression in large and small tissue specimens between the E1L3N and 22C3 assays
| Large (n = 185) | Small (n = 114) | p value | |
|---|---|---|---|
| E1L3N | |||
| Negative | 125 (67.6%) | 68 (59.6%) | |
| Weak positive | 40 (21.6%) | 25 (21.9%) | 0.160 |
| Positive | 20 (10.8%) | 21 (18.4%) | |
| 22C3 | |||
| Negative | 127 (68.6%) | 66 (57.9%) | |
| Weak positive | 38 (20.5%) | 26 (22.8%) | 0.080 |
| Positive | 20 (10.8%) | 22 (19.3%) | |
| Overall agreement | 97.8% (94.6–99.4%) a | 93.9% (87.8–97.5%) | 0.076 |
95% confidence interval.
4. DISCUSSION
Immunotherapy brings a revolutionary change for cancer treatment. 22 , 23 At present, the expression of PD‐L1 detected by IHC has been successfully used to predict the response to anti‐PD‐1/PD‐L1 inhibitors. 24 With the increase of immune checkpoint inhibitors, different antibodies, clones, and platforms were developed for a specific inhibitor. 25 For example, only PD‐L1 IHC staining using 22C3 antibody under the Dako platform is approved as a diagnostic assay by FDA for NSCLC patients with pembrolizumab. 26 However, of note, Marchetti et al. 27 found a high concordance between the 22C3 and SP263 assays for detecting PD‐L1 expression in NSCLC patients. Yet, the Dako and Ventana platforms are not universally available and still expensive. Thus, it is of great importance and interest to evaluate the diagnostic performance of laboratory‐developed tests (LDTs) for PD‐L1 expression.
E1L3N has been proposed as one of the most widely used and affordable LDTs for evaluating PD‐L1 expression. Adam et al. 9 performed a multicenter study to compare PD‐L1 standardized assays with LDTs and found that E1L3N can achieve high concordance with 28‐8, 22C3, and SP263. Munari et al. 19 also reported that E1L3N had a high concordance with SP263 but a low concordance with 22C3 at both 1% and 50% cutoffs. Moreover, 100% sensitivity was obtained for E1L3N when compared with both SP263 and 22C3 at 50% cutoff. 19 In our study, relative to the 22C3 assay, the negative coincidence rate (<1%), weak positive coincidence rate (1%‐49%), and positive coincidence rate (≥50%) of PD‐L1 expression detected with the E1L3N assay were 97.4%, 92.2%, and 97.6%, respectively. Moreover, an overall agreement of 96.3% was achieved for assaying PD‐L1 expression in tumor tissues between the E1L3N and 22C3 assays. We also found that the expression of PD‐L1 was not detected in tumor tissues of benign lung disease using these two assays, suggesting a high specificity for PD‐L1 testing.
In general, only histological samples especially large tissues are used to quantify PD‐L1 expression for immunotherapy in clinical trials. 28 In clinical practice, however, large histological samples are difficult to be obtained in NSCLC patients especially at advanced stage, so small specimens collected by bronchoscopy can be a good choice. Thus, many researchers have explored the feasibility and effectiveness of small biopsy specimens for PD‐L1 detection. For example, Heymann et al. 29 evaluated 214 specimens including cytology, small biopsies, and surgically resected specimens from 188 NSCLC patients for evaluating PD‐L1 expression and found that there were no significant differences in the PD‐L1 positive rate between resection and small biopsy specimens (p = 0.083). Sakakibara et al. 30 also reported that cytological specimens from EBUS‐TBNA had the advantages of high consistency with histological specimens and minimal invasion. A study performed by Skov et al. 31 revealed that 86 pairs of cytological wax blocks obtained from the same part of the lung tissue were highly correlated with histological specimens when using the 22C3 and 28‐8 assays for PD‐L1 detection. For the detection of PD‐L1, in addition, high concordance between paired cytological smears and formalin‐fixed paraffin‐embedded samples of NSCLC patients was also observed using both the 22C3 and SP263 assays. 32 In the present study, we compared the performance of PD‐L1 expression detection in large and small tissue samples of NSCLC patients between the E1L3N and 22C3 assays. The results demonstrate that the overall concordances were 97.8% and 93.9% for large and small specimens, respectively, and no statistically significant difference was obtained between these two assays (p = 0.076). These findings indicate that E1L3N is qualified for detecting PD‐L1 expression in both large and small tissue specimens of NSCLC patients.
5. CONCLUSIONS
In this study, we found a high consistency for the detection of PD‐L1 in tumor tissues of NSCLC patients between the E1L3N and 22C3 assays, which is independent of the sample size. Moreover, these two assays exhibited a high specificity for PD‐L1 testing. Therefore, E1L3N might be a reliable alternative antibody to assess PD‐L1 expression status for NSCLC patients. To our knowledge, this is the first study to evaluate the performance of PD‐L1 detection in NSCLC patients using clone E1L3N produced by Amoy Diagnostics. However, further large‐scale and multicenter assessments still need to confirm our conclusions.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
YPL, CSC, and GRC contributed to the experimental design. HTX, JRY, and YPL contributed to clinical diagnosis and sample collection. HXZ, TTH, and XDD contributed to PD‐L1 testing. YPL and HYX contributed to the data analysis, result interpretation, and writing. All authors have reviewed and approved the final study.
Hanyan Xu and Xidan Dong contributed equally to this work.
Funding information
This study was supported by Wenzhou Municipal Science and Technology Bureau (No. ZH2017001). The authors thank all the patients who participated in this study.
Contributor Information
Junru Ye, Email: yejunru0414@163.com.
Yuping Li, Email: wzliyp@163.com.
DATA AVAILABILITY STATEMENT
The data used in the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. [DOI] [PubMed] [Google Scholar]
- 2. Duma N, Santana‐Davila R, Molina JR. Non‐small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623‐1640. [DOI] [PubMed] [Google Scholar]
- 3. Mok TSK, Wu Y‐L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393:1819‐1830. [DOI] [PubMed] [Google Scholar]
- 4. Pacheco JM. KEYNOTE‐407: changing the way we treat stage IV squamous non‐small cell lung cancer. Transl Lung Cancer Res. 2020;9:148‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD‐L1 expression in lung cancer. J Thorac Oncol. 2016;11:964‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lantuejoul S, Damotte D, Hofman V, Adam J. Programmed death ligand 1 immunohistochemistry in non‐small cell lung carcinoma. J Thorac Dis. 2019;11:S89‐S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirsch FR, McElhinny A, Stanforth D, et al. PD‐L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD‐L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208‐222. [DOI] [PubMed] [Google Scholar]
- 8. Rimm DL, Han G, Taube JM, et al. A prospective, multi‐institutional, pathologist‐based assessment of 4 immunohistochemistry assays for PD‐L1 expression in non–small cell lung cancer. JAMA Oncol. 2017;3:1051‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD‐L1 IHC testing in non‐small‐cell lung cancer. Ann Oncol. 2018;29:953‐958. [DOI] [PubMed] [Google Scholar]
- 10. Munari E, Rossi G, Zamboni G, et al. PD‐L1 assays 22C3 and SP263 are not interchangeable in non–small cell lung cancer when considering clinically relevant cutoffs. Am J Surgic Pathol. 2018;42:1384‐1389. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, He HS, Hu QF, Wang G. Adrenal diffuse large B‐cell lymphoma with high PD‐L1 expression: two case reports and literature review. J Clin Lab Anal. 2020;34:e23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Junttila A, Helminen O, Väyrynen JP, et al. Immunophenotype based on inflammatory cells, PD‐1/PD‐L1 signalling pathway and M2 macrophages predicts survival in gastric cancer. Bri J Cancer. 2020;123:1625‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aggen DH, Drake CG, Rini BI. Targeting PD‐1 or PD‐L1 in metastatic kidney cancer: combination therapy in the first‐line setting. Clin Cancer Res. 2020;26:2087‐2095. [DOI] [PubMed] [Google Scholar]
- 14. Rao Q, Li M, Xu W, et al. Clinical benefits of PD‐1/PD‐L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and meta‐analysis. Hepatol Int. 2020;14:765‐775. [DOI] [PubMed] [Google Scholar]
- 15. Büttner R, Gosney JR, Skov BG, et al. Programmed death‐ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non–small‐cell lung cancer. J Clin Oncol. 2017;35:3867‐3876. [DOI] [PubMed] [Google Scholar]
- 16. Kim H, Kwon HJ, Park SY, et al. PD‐L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non‐small cell lung cancer: a comparative study. Oncotarget. 2017;8:98524‐98532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parra ER, Villalobos P, Mino B, Rodriguez‐Canales J. Comparison of different antibody clones for immunohistochemistry detection of programmed cell death ligand 1 (PD‐L1) on non–small cell lung carcinoma. App Immunohistochem Mol Morphol. 2018;26:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cogswell J, Inzunza HD, Wu Q, et al. An analytical comparison of Dako 28‐8 pharmDx assay and an E1L3N laboratory‐developed test in the immunohistochemical detection of programmed death‐ligand 1. Mol Diagn Ther. 2017;21:85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munari E, Zamboni G, Lunardi G, et al. PD‐L1 expression in non–small cell lung cancer: evaluation of the diagnostic accuracy of a laboratory‐developed test using clone E1L3N in comparison with 22C3 and SP263 assays. Hum Pathol. 2019;90:54‐59. [DOI] [PubMed] [Google Scholar]
- 20. Smith J, Robida MD, Acosta K, et al. Quantitative and qualitative characterization of two PD‐L1 clones: SP263 and E1L3N. Diagn Pathol. 2016;11:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39‐51. [DOI] [PubMed] [Google Scholar]
- 22. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD‐L1 checkpoint. Immunity. 2018;48:434‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gridelli C, Ascierto PA, Barberis MCP, et al. Immunotherapy of non‐small cell lung cancer: report from an international experts panel meeting of the Italian association of thoracic oncology. Exp Opin Biol Ther. 2016;16:1479‐1489. [DOI] [PubMed] [Google Scholar]
- 26. Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD‐1/PD‐L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marchetti A, Barberis M, Franco R, et al. Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD‐L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J Thorac Oncol. 2017;12:1654‐1663. [DOI] [PubMed] [Google Scholar]
- 28. Jin Y, Zhao J, Shi X, Yu X. Prognostic value of programed death ligand 1 in patients with solid tumors: a meta‐analysis. J Cancer Res Therap. 2015;11:38. [DOI] [PubMed] [Google Scholar]
- 29. Heymann JJ, Bulman WA, Swinarski D, et al. PD‐L1 expression in non‐small cell lung carcinoma: comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol. 2017;125:896‐907. [DOI] [PubMed] [Google Scholar]
- 30. Sakakibara R, Inamura K, Tambo Y, et al. EBUS‐TBNA as a promising method for the evaluation of tumor PD‐L1 expression in lung cancer. Clin Lung Cancer. 2017;18:527‐534. [DOI] [PubMed] [Google Scholar]
- 31. Skov BG, Skov T. Paired comparison of PD‐L1 expression on cytologic and histologic specimens from malignancies in the lung assessed with PD‐L1 IHC 28‐8pharmDx and PD‐L1 IHC 22C3pharmDx. App Immunohistochem Mol Morphol. 2017;25:453‐459. [DOI] [PubMed] [Google Scholar]
- 32. Lozano MD, Abengozar‐Muela M, Echeveste JI, et al. Programmed death–ligand 1 expression on direct Pap‐stained cytology smears from non–small cell lung cancer: comparison with cell blocks and surgical resection specimens. Cancer Cytopathol. 2019;127:470‐480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in the current study are available from the corresponding author upon reasonable request.


