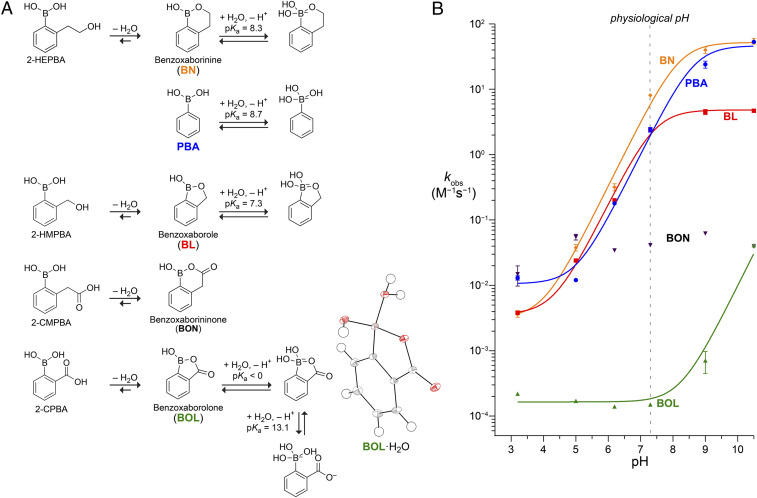
Fig. 2.
(A) Structures of relevant hydration and protonation states and pKa values of 2-HEPBA: BN (69), PBA (70), 2-HMPBA: BL (69), 2-CMPBA; BON, and 2-CPBA; BOL (this work). (Inset) Oak Ridge thermal ellipsoid plot diagram of hydrated BOL from X-ray crystallography. (B) pH dependence of the observed second-order rate constant for the oxidation of boronic acids by hydrogen peroxide. Data were fitted to the equation kobs = k1 + k2/(1 + 10pKa − pH) by using pKa values in A to give k1 = (3.2 ± 0.6) × 10−3 M−1·s−1, k2 = 59 ± 5 M−1·s−1 for BN; k1 = (1.1 ± 0.2) × 10−2 M−1 · s−1, k2 = 49 ± 4 M−1 · s−1 for PBA; k1 = (3.4 ± 0.4) × 10−3 M−1 · s−1, k2 = 4.2 ± 0.2 M−1 · s−1 for BL; and k1 = (1.6 ± 0.1) × 10−4 M−1 · s−1, k2 = 9.7 ± 1.3 M−1 · s−1 for BOL. Data for BON show no pH dependence with a mean kobs = 0.041 ± 0.017 M−1 · s−1.