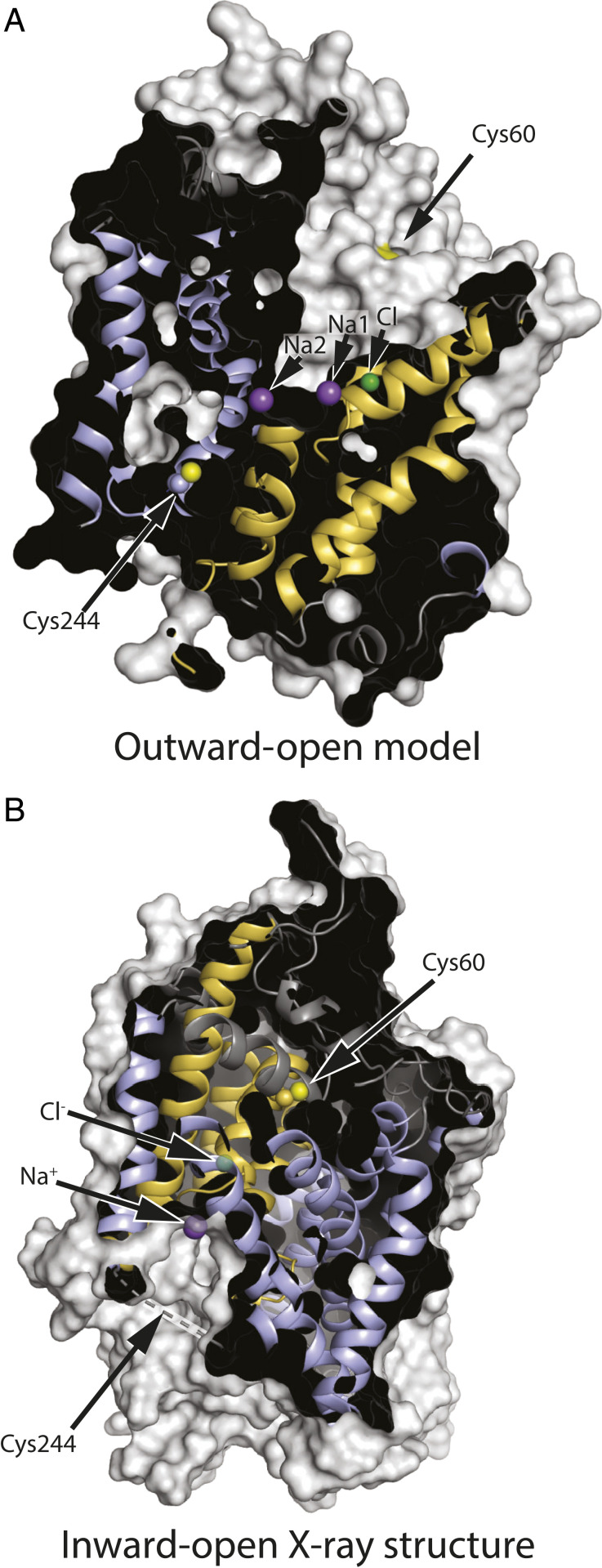
Fig. 3.
GlyT1 in outward-open (model) and inward-open (X-ray structure) conformations. (A) In this outward-open model (SI Appendix, Fig. S1) showing, for illustration, cysteine replacing both Tyr60 and Ser244, Cys60 is accessible through the extracellular pathway, but Cys244 is buried. (B) In the inward-open conformation (PDB ID 6ZPL with the ligand removed) (44), the closed extracellular pathway buries Cys60 within the protein structure. Cys244 is in an unresolved region, shown by the dashed line, which is expected to be accessible to the cytoplasm. Residue numbering is based on the published sequence of GlyT1b (43). Helices from the bundle domain are shown in gold, and those from the scaffold domain are light blue, with cysteine sulfhydryls in yellow. Positions for the ion binding sites were superimposed on the model, and ions found in the inward-open structure are superimposed in semitransparent form.